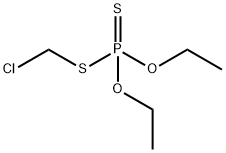
CHLORMEPHOS
- CAS No.
- 24934-91-6
- Chemical Name:
- CHLORMEPHOS
- Synonyms
- DOTAN;MC2188;P 2188;ai3-25533;CHLORMEFOS;CHLORMEPHOS;caswellno195b;Chlormethylfos;chlormephos(bsi,iso);CHLORMEPHOS STANDARD
- CBNumber:
- CB4364182
- Molecular Formula:
- C5H12ClO2PS2
- Molecular Weight:
- 234.7
- MDL Number:
- MFCD00055471
- MOL File:
- 24934-91-6.mol
| Melting point | 25°C |
|---|---|
| Boiling point | bp1mm 93-95°; bp0.1 Torr 81-85° |
| Density | d20 1.260 |
| vapor pressure | 7.6 Pa (30 °C) |
| refractive index | nD220 1.5244 |
| Flash point | 100 °C |
| storage temp. | 0-6°C |
| Water Solubility | 60 mg l-1(20 °C) |
| form | liquid |
| EWG's Food Scores | 4-5 |
| FDA UNII | 1N10F47JUS |
| EPA Substance Registry System | Chlormephos (24934-91-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300+H310-H410 |
| Precautionary statements | P273-P280-P301+P310+P330-P302+P352+P310-P391-P501 |
| Hazard Codes | T+,N |
| Risk Statements | 27/28-50/53 |
| Safety Statements | 28-36/37-45-60-61-27 |
| RIDADR | 3018 |
| RTECS | TD5170000 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| Toxicity | LD50 in rats (mg/kg): 7 orally; 27 dermally; LC50 in fish: 1.5 mg/l (Lynch) |
CHLORMEPHOS Chemical Properties,Uses,Production
Description
Chlormephos is an organophosphate, colorlessliquid. Molecular weight=234.70; Boiling point =81-85℃ at 0.1 mmHg. Hazard Identification (based onNFPA 704 M Rating System): Health 3, Flammability 2,Reactivity 0. Soluble in water; solubility=60 mg/L at20℃.
Uses
Insecticide primarily for soil applications.
Uses
Chlormefos has agricultural applications. Useful for preparing compositions for controlling plant diseases.
Uses
Chlormephos is used to control soil insects such as wireworms in a number of crops.
Definition
ChEBI: Chlormephos is an organic thiophosphate, an organothiophosphate insecticide and an organochlorine insecticide. It has a role as an agrochemical and an EC 3.1.1.7 (acetylcholinesterase) inhibitor.
General Description
Colorless liquid. Used as a soil insecticide. Not registered as a pesticide in the U.S.
Air & Water Reactions
Slightly soluble in water [Farm Chemicals Handbook].
Reactivity Profile
Organophosphates, such as CHLORMEPHOS, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
Health Hazard
(Non-Specific -- Organophosphorus Pesticide, Liquid, n.o.s.) CHLORMEPHOS is poisonous; it may be fatal if inhaled, swallowed, or absorbed through the skin.
Fire Hazard
(Non-Specific -- Organophosphorus Pesticide, Liquid, n.o.s.) Container may explode in heat of fire. Fire and runoff from control water may produce irritating or poisonous gases.
Potential Exposure
This material is a soil insecticide. Notregistered as a pesticide in the US.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If this chemicalhas been inhaled, remove from exposure, begin rescuebreathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit. Medical observation isrecommended for 24-48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Metabolic pathway
Chlormephos is a soil insecticide with considerable vapour phase activity. The main soil metabolite is ethion. The mechanism of hydrolysis of chlormephos is mainly by the nucleophilic attack of OH- on the Smethylene carbon to yield O,O-diethyl phosphorodithioate and in this respect it is similar to mecarbam. This mechanism may afford ethion when the nucleophile is O,O-diethyl phosphorodithioate. The other mechanism of hydrolysis is by attack on phosphorus to yield O,O-diethyl phosphorothioate and these two routes of hydrolytic degradation apparently dominate the metabolism of the insecticide.
Metabolism
In soils, chlormephos is converted to ethion by reaction with the hydrolytic product, O,O-diethyl hydrogen phosphorothioate.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working withChlormephos you should be trained on its proper handlingand storage. Should be protected from moisture and storedin glass-lined or polyethylene-lined containers. Keep awayfrom strong oxidizers. Sources of ignition, such as smokingand open flames, are prohibited where this chemical isused, handled, or stored in a manner that could create apotential fire or explosion hazard.
Shipping
This chemical requires a shipping label of“POISONOUS/TOXIC MATERIALS” for organophosphorus pesticides, liquid, toxic. It falls in Hazard Class6.1.
Toxicity evaluation
The acute oral LD50 for rats is 7 mg/kg. NOEL (90 d) for rats is 0.39 mg/kg diet (0.002 mg/kg/d). In rats, orally administered chlormephos is almost completely eliminated within 24 h in the urine as diethyl hydrogen phosphate and O,O-diethyl hydrogen phosphorothioate.
Degradation
In strongly basic solution (0.5 M aqueous KOH), hydroxide ion attack on chlormephos may occur on the phosphorus atom with P-C bond cleavage to yield O,O-diethyl phosphorothioate (2) or on the S-methylene carbon atom to give O,O-diethyl phosphorodithioate (3), both of which may react with chlormephos to give further products. Hudson et al. (1991) showed that both hydrolysis products 2 and 3 could react with chlormephos via attact on the ethoxy group to yield the transethylation products O,O,Striethyl phophorothioate (4) and O,O,S-triethyl phophoroditluoate (5) respectively. Compound 3 could also attack the phosphorus atom of chlormephos to yield O,O,O’,O’-tetraethyl trithiopyrophosphate (6) and the S-methylene carbon to give ethion (7). Reaction products were identified by GC-MS after conversion of the acidic products into their methyl esters by reaction with diazomethane. Routes by which these products may be formed in strongly basic solution are shown in Scheme 1.
Incompatibilities
Contact with oxidizers may cause therelease of phosphorous oxides. Contact with strong reducingagents, such as hydrides, may cause the formation of flammable and toxic phosphine gas. Contact with alkaline materials causes rapid hydrolysis. May corrode metals.