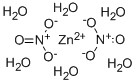Zinc nitrate hexahydrate
- CAS No.
- 10196-18-6
- Chemical Name:
- Zinc nitrate hexahydrate
- Synonyms
- Zinknitrat;ZincNitrateAr;ZINC NITRATE 6H2O;dusicnanzinecnaty;ZINC NITRATE HYDRATE;ZINC NITRATE 99.999%;ZINC NITRATE HYDRATED;ZINC NITRATE, HYDROUS;ZINC NITRATE 6HYD XTL;Zinc nitrate, GR 98%+
- CBNumber:
- CB4421557
- Molecular Formula:
- H12N2O12Zn
- Molecular Weight:
- 297.49
- MDL Number:
- MFCD00149889
- MOL File:
- 10196-18-6.mol
- MSDS File:
- SDS
| Melting point | 36 °C(lit.) |
|---|---|
| Density | 2.065 g/mL at 25 °C(lit.) |
| solubility | water: soluble(lit.) |
| form | Solid |
| Specific Gravity | 2.07 |
| color | White |
| PH | pH(100g/l, 25℃) : 3.5~5.4 |
| Water Solubility | 1800 G/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,10144 |
| InChIKey | JGPSMWXKRPZZRG-UHFFFAOYSA-N |
| CAS DataBase Reference | 10196-18-6(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H302-H315-H319-H335-H410 | |||||||||
| Precautionary statements | P210-P220-P273-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | O,Xn | |||||||||
| Risk Statements | 8-22-36/37/38-38 | |||||||||
| Safety Statements | 17-26-24/25 | |||||||||
| RIDADR | UN 1514 5.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | ZH4775000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28342990 | |||||||||
| NFPA 704 |
|
Zinc nitrate hexahydrate price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 228737 | Zinc nitrate hexahydrate reagent grade, 98% | 10196-18-6 | 100g | $27.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 228737 | Zinc nitrate hexahydrate reagent grade, 98% | 10196-18-6 | 500g | $80.6 | 2024-03-01 | Buy |
| Alfa Aesar | 011136 | Zinc nitrate hexahydrate, Puratronic? 99.998% (metals basis) | 10196-18-6 | 50g | $278 | 2024-03-01 | Buy |
| Alfa Aesar | 011136 | Zinc nitrate hexahydrate, Puratronic? 99.998% (metals basis) | 10196-18-6 | 250g | $808 | 2023-06-20 | Buy |
| Strem Chemicals | 93-3015 | Zinc nitrate hexahydrate, min. 98% | 10196-18-6 | 100g | $23 | 2024-03-01 | Buy |
Zinc nitrate hexahydrate Chemical Properties,Uses,Production
Physical Properties
The hexahydrate, Zn(NO3)2•6H2O, is a colorless and odorless crystalline solid; tetragonal structure; density 2.065 g/cm3 at 15°C; melts at 36.4°C; loses all its water of crystallization between 105 to 131°C; very soluble in water, about 184 g/100mL water at 20°C; the aqueous solution acidic, the pH of a 5% solution is about 5.1; also very soluble in alcohol.
The trihydrate, Zn(NO3)2•3H2O consists of colorless needles; melts at 45.5°C; very soluble in water, 327 g/100mL at 40°C.
Uses
The compound is used as a mordant in dyeing and as a latex coagulant. It also is used as an acid catalyst and as an analytical standard for zinc.
Preparation
Zinc nitrate is prepared by reacting zinc metal, zinc oxide or zinc hydroxide with nitric acid followed by crystallization. The salt is obtained as hexahydrate:
Zn + 2HNO3 → Zn(NO3)2 + H2
ZnO + 2HNO3 → Zn(NO3)2 + H2O
Zn(OH)2 + 2HNO3 → Zn(NO3)2 + 2H2O
The salt also is sold commercially in the form of fused pieces and technical flakes containing about 20% and 25.6% water, respectively.
Chemical Properties
White crystal
Uses
Zinc Nitrate Hexahydrate is a component used in the composition for nourishing and protecting fruit trees from acid rain.
Uses
Used as a mordant in dyeing and chemical synthesis of coordination polymers
Uses
Zinc nitrate hexahydrate is used in the synthesis of coordination polymers. It is utilized in the preparation of zinc oxide based nanowires. It is also used as a mordant in dyeing. It is involved in the preparation of a new microporous, organically templated zinc phosphate, which finds application as catalysts and other applications involving microporosity.
Zinc nitrate hexahydrate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 | sales@simchoicechem.com | China | 255 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| SINOPRO.CO.LTD | 0082-42-721-7177 | michael@sinopro.co.kr | South Korea | 298 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9409 | 58 |
View Lastest Price from Zinc nitrate hexahydrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-07-25 | Zinc Nitrate Hexahydrate
10196-18-6
|
US $3.00 / Kg/Drum | 20T | 80%;98% | 3000tons/month | Hebei Yanxi Chemical Co., Ltd. | |
 |
2021-07-02 | Zinc nitrate hexahydrate
10196-18-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2019-07-12 | Zinc nitrate hexahydrate
10196-18-6
|
US $1.00 / KG | 1KG | 99% | 25kg | Career Henan Chemical Co |
-

- Zinc Nitrate Hexahydrate
10196-18-6
- US $3.00 / Kg/Drum
- 80%;98%
- Hebei Yanxi Chemical Co., Ltd.
-

- Zinc nitrate hexahydrate
10196-18-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Zinc nitrate hexahydrate
10196-18-6
- US $1.00 / KG
- 99%
- Career Henan Chemical Co