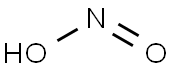
NITROUS ACID
- CAS No.
- 7782-77-6
- Chemical Name:
- NITROUS ACID
- Synonyms
- HNO2;HONO;nitrousaci;NITROUS ACID;kyselinadusite;Kyselina dusite;Nitrosyl hydroxide;Nitrous acid, trans
- CBNumber:
- CB4453949
- Molecular Formula:
- HNO2
Lewis structure
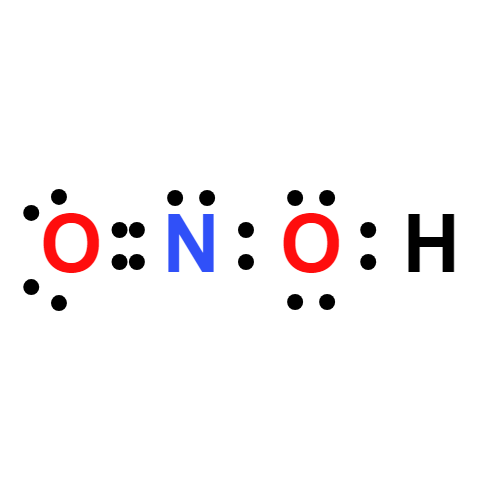
- Molecular Weight:
- 47.01
- MDL Number:
- MFCD01310438
- MOL File:
- 7782-77-6.mol
| Density | 1.54±0.1 g/cm3(Predicted) |
|---|---|
| form | stable only in solution |
| pka | pK (25°) 3.35 |
| color | stable only in solution |
| PH | 3.28(1 mM solution);2.67(10 mM solution);2.13(100 mM solution) |
| EWG's Food Scores | 1 |
| FDA UNII | T2I5UM75DN |
| EPA Substance Registry System | Nitrous acid (7782-77-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H314 |
| Precautionary statements | P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501 |
NITROUS ACID price More Price(1)
NITROUS ACID Chemical Properties,Uses,Production
Chemical Properties
Nitrous acid,HN02, is the aqueous solution of nitrogen trioxide. It is a moderately strong and rapid oxidizing agent used for diazotization.
Uses
Nitrous acid is a diazotizing agent. The acid diazotizes primary aromatic amines to diazo derivatives in manufacturing azo dyes.
Reactions
Nitrous acid is unstable. It decomposes to form nitric acid and nitric oxide:
3HNO2 → NO3¯ + H3O+ + 2NO
Strong oxidizing agents, such as permanganate, readily oxidize nitrous acid to nitric acid.
Nitrous acid is an effective oxidizing agent. It oxidizes hydrogen sulfide to sulfur forming either nitric oxide or ammonia, depending on the acidity of the solution:
2HNO2 + H2S → S + 2NO + 2H2O
HNO2 + 3H2S → 3S + NH3 + 2H2O
In acid medium it oxidizes iodide ion to iodine:
HNO2 + I¯ + 6H+ → 3I2 + NH3 + 2H2O
Description
Nitrous acid (molecular formula?HNO2) is a weak
and monobasic acid known only in solution and in the
form of nitrite salts. Nitrous acid rapidly decomposes
into nitrogen oxide, nitric oxide and water when in
solution:
2HNO2 ? NO2 + NO+H2O
It can also decompose into nitric acid and nitrous
oxide and water.
4HNO2 ? 2HNO3 +N2O +H2O
Nitric acid (HNO3), also known as “aqua fortis”
and “spirit of nitre”, is a highly corrosive and toxic
strong acid that can cause severe burns. It is colorless
when pure and a slight yellow when “glacial”. Older
samples tend to acquire a yellow cast due to the accumulation
of various oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as
“fuming nitric acid”.
Chemical Properties
A weak acid occurring only in the form of a light-blue solution.
Physical properties
Pale blue solution; stable only in solution; weak acid, Ka 4.5x10-4.
Uses
Formation of diazotizing compounds by reaction with primary aromatic amines, source of nitric oxide.
Uses
Nitrous acid is a nitrogen oxoacid. It is a conjugate acid of a nitrite. It (as sodium nitrite) is used as part of an intravenous mixture with sodium thiosulfate to treat cyanide poisoning. There is also research to investigate its applicability towards treatments for heart attacks, brain aneurysms, pulmonary hypertension in infants, and Pseudomonas aeruginosa infections.
Preparation
Nitrous acid may be obtained in solution by adding a strong acid to nitrite; e.g., adding hydrochloric acid to sodium nitrite solution:
H+ + NO2 ˉ → HNO2.
Definition
A weak acid known only in solution, obtained by acidifying a solution of a nitrite. It readily decomposes on warming or shaking to nitrogen monoxide and nitric acid. The use of nitrous acid is very important in the dyestuffs industry in the diazo reaction: nitrous acid is liberated by acidifying a solution of a nitrite (usually sodium nitrite) in the presence of the compound to be diazotized. Nitrous acid and the nitrites are normally reducing agents but in certain circumstances they can behave as oxidizing agents, e.g. with sulfur dioxide and hydrogen sulfide.
Definition
nitrous acid: A weak acid, HNO2,known only in solution and in thegas phase.It is prepared by the actionof acids upon nitrites, preferablyusing a combination that removesthe salt as an insoluble precipitate(e.g.Ba(NO2)2 and H2SO4). The solutionsare unstable and decompose on heating to give nitric acid and nitrogenmonoxide.Nitrous acid can functionboth as an oxidizing agent(forms NO) with I– and Fe2+, or as areducing agent (forms NO3-) with,forexample, Cu2+; the latter is mostcommon.It is widely used (preparedin situ) for the preparation of diazoniumcompounds in organic chemistry.The full systematic name isdioxonitric(III) acid.
Hazard
Rapidly forms nitric oxide and nitric acid in water; [Merck Index] A strong oxidizer; Causes burns; Highly toxic by ingestion and inhalation.
Safety Profile
Mutation data reported. Flammable by chemical reaction; a powerful oxidizer. Explodes on contact with phosphorus trichloride. Reacts violently with PH3 and Pcb. Reactions with l-amino- 5-nitrophenol, ammonium decahydroborate(2-), hydrazine (product is hydrogen azide) may give explosive products. Incompatible with anilines (e.g., 4- bromoahe , 2-chloroaniline, 3- chloroaniline, 2-nitroadine, 3-nitroaniline, 4-nitroaniline, aniline ), semicarbazone, silver nitrate. When heated to decomposition it emits hghly toxic fumes of NOx. See also NITRIC OXIDE.
NITROUS ACID Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2739 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Golden Pharma Co., Limited | +undefined18958062155 | sales@zjgoldpharm.com | China | 5905 | 58 |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 027-88013699 17354350817 | Ryan@jiutian-bio.com | China | 7433 | 58 |
| Jiangsu Aikon Biopharmaceutical R&D co.,Ltd. | 025-66113011 18112977050 | cb6@aikonchem.com | China | 16687 | 50 |
| Lynnchem | 86-(0)29-85992781 17792393971 | info@lynnchem.com | China | 4587 | 58 |
| Novachemistry | 44-20819178-90 02081917890 | info@novachemistry.com | United Kingdom | 4381 | 58 |
| Lianyungang Xuanyuan Chemical Co., Ltd | +86-0518-85415818 18000194506 | 18000194506@qq.com | China | 309 | 55 |
| Rhawn Reagent | 400-400-1332688 18019345275 | amy@rhawn.cn | China | 15503 | 58 |
| Hubei yongkuo Technology Co., Ltd | 027-59223108 15972152991 | 1248580099@qq.com | China | 10007 | 58 |
7782-77-6(NITROUS ACID)Related Search:
1of4