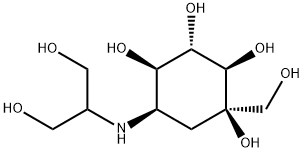
Voglibose
- CAS No.
- 83480-29-9
- Chemical Name:
- Voglibose
- Synonyms
- 5-(1,3-dihydroxypropan-2-ylamino)-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetrol;Basen;Jumeal;AO 128;A 71100;Glustat;Beigrace;Voglistat;VOGLIBOSE;Voglibose D5
- CBNumber:
- CB4481287
- Molecular Formula:
- C10H21NO7
- Molecular Weight:
- 267.28
- MDL Number:
- MFCD00865496
- MOL File:
- 83480-29-9.mol
- MSDS File:
- SDS
| Melting point | 162-163°C |
|---|---|
| alpha | D25 +26.2° (c = 1 in water) |
| Boiling point | 601.9±55.0 °C(Predicted) |
| Density | 1.58±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Very Slightly, Heated), Water (Sparingly, Sonicated) |
| form | Solid |
| pka | 13.26±0.70(Predicted) |
| color | White to Off-White |
| Merck | 14,10029 |
| InChIKey | FZNCGRZWXLXZSZ-CIQUZCHMSA-N |
| CAS DataBase Reference | 83480-29-9(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | S77P977AG8 |
| NCI Drug Dictionary | Basen |
| ATC code | A10BF03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 20/21/22-36/37/38 |
| Safety Statements | 36/37-37/39-26 |
| WGK Germany | 3 |
| RTECS | NM7524600 |
| HS Code | 2940.00.6000 |
Voglibose price More Price(39)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 50359 | Voglibose ≥97.0% (TLC) | 83480-29-9 | 10mg | $305 | 2024-03-01 | Buy |
| TCI Chemical | V0119 | Voglibose >98.0%(HPLC)(T) | 83480-29-9 | 100mg | $170 | 2024-03-01 | Buy |
| Cayman Chemical | 14179 | Voglibose ≥95% | 83480-29-9 | 10mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 14179 | Voglibose ≥95% | 83480-29-9 | 50mg | $93 | 2024-03-01 | Buy |
| Cayman Chemical | 14179 | Voglibose ≥95% | 83480-29-9 | 100mg | $139 | 2024-03-01 | Buy |
Voglibose Chemical Properties,Uses,Production
Oral hypoglycemic agents
Voglibose is an oral hypoglycemic agents which can alleviate the postprandial hyperglycemia of diabetes patients. It is first successfully developed by the Takeda Company in Japan. It belongs to α-glucosidase inhibitor (a drug which can delay the intestinal absorption of carbohydrates to achieve hypoglycemic effect). Its mechanism of action is through inhibiting the activity of disaccharides hydrolase (α-glucosidase) which can hydrolyze disaccharide into monosaccharide inside the gut and thus delaying the digestion and absorption of sugar and further alleviating the postprandial hyperglycemia. In the assay of measuring the exhaled hydrogen of healthy adults given sucrose loading, the results have confirmed that this drug in its clinical dosage has an inhibitory effect on hyperglycemia. When normal rats are subject to oral administration, this product can inhibit the increased blood sugar level after the increased loading of starch, sucrose, glucose and maltose, while it has no inhibitory effect on the increased blood sugar after increased loading of glucose, fructose, and lactose. In vitro analysis of mechanism of action have shown that, for the maltase and sucrose obtained from the small intestine of pig and rat, this drug has a strong inhibitory effect; on the other hand, it has a relative low inhibitory effect on the pancreatic α-amylase of pig and rat and has no inhibitory effect on β-glucosidase. It has a competitive inhibitory effect on the disaccharide hydrolase of the isomaltase-sucrase complex from the rat small intestine.
It is understood that voglibose has a broad scope of application; it is not only suitable for elderly patients with diabetes, but also has significant efficacy on patients with refractory type II diabetes, patients with secondary failure of sulfonylureas, patients with combined hyperlipidemia diabetes, patients with merged cardiovascular diabetes disease as well as patients with combined hyperinsulinemia. Because this product does not stimulate the secretion of insulin, it can reduce the harmful effects of postprandial hyperinsulinemia, reducing the resistance of insulin and contributes to the prevention and treatment of metabolic syndrome such as cardiovascular complications.
Side Effects
Mechanism of occurrence of side effects of oral administration of hypoglycemic agents is generally that: when the unabsorbed carbohydrate enters into the large intestine, under the action of bacteria, it subjects to glycolysis which produces carbon dioxide, hydrogen, and organic acids. At the same time, because of the increased osmotic pressure of intestinal, water retention happen which therefore causes increased exhausting, bloating, and diarrhea. Since voglibose can selectively inhibit the activity of the disaccharide hydrolase in the intestine, further delaying the rapid digestion and absorption of carbohydrate in the upper small intestine, and thus reducing saccharide, leading to a lower incidence of adverse reactions. It is known to have the following adverse reactions:
1. The digestive system: diarrhea, loose stools, borborygumus, abdominal pain, constipation, loss of appetite, nausea, vomiting, heartburn (incidence of 0.1 to 5%), stomatitis, thirst, abnormal taste, and pneumatosis cystoides gastrointestinalis (0.1%).
2. Allergic symptoms: rash, itching, light sensitivity (incidence of 0.1% or less).
3. Liver: GOT, GPT, LDH, γ-GTP, ALP are increased (0.1% incidence).
4. The nervous system: headache, dizziness, staggering, drowsiness (0.1% incidence).
5. The blood system: anemia (incidence of 0.1 to 5%), thrombocytopenia (0.1%).
6. Other: paralysis, facial swelling, hazy eyes, fever, flu, tiredness, feeling of fatigue, hyperkalemia, rise of serum amylase, decrease of high-density lipoprotein, sweating, hair loss (incidence of 0.1%).
The above information is edited by the chemicalbook of Dai Xiongfeng.
Uses
1. It is used for treating diabetes.
2. Anti-diabetic drug.
Description
Voglibose was introduced in Japan for the treatment of postprandial hyperglycemia in diabetic patients. As an orally active α-D-glucosidase inhibitor, it is effective in decreasing the release of glucose from carbohydrate ingestion and slowing the increase of postprandial blood glucose levels, as demonstrated in animals and humans following sucrose and starch loading. In addlion, voglibose was reported to have prophylactic effect in genetically obese Wistar rats, maintaining low plasma levels of glucose, triglyceride and insulin, indicating a potential usefulness for the management of other carbohydrate-dependent metabolic disorders such as obesity. Voglibose is much more potent and has fewer side effects than acarbose, another agent in its class.
Chemical Properties
Colourless Crystalline Solid
Originator
Takeda (Japan)
Uses
For the treatment of diabetes. It is specifically used for lowering post-prandial blood glucose levels thereby reducing the risk of macrovascular complications.
Uses
An α-Glucosidase inhibitor used as an antidiabetic.
Uses
An alpha-Glucosidase inhibitor used as an antidiabetic
Uses
Voglibose is an α-glucosidase inhibitor, similar to acarbose and miglitol, used for lowering post-prandial hyperglycemia (PPHG) in people with diabetes mellitus. Voglibose is used to study it benefits as a protectant against ischemia-reperfusion injury through glucagon-like peptide 1 receptors and the phosphoinositide 3-kinase-Akt-endothelial nitric oxide synthase pathway.
Definition
ChEBI: Voglibose is an organic molecular entity.
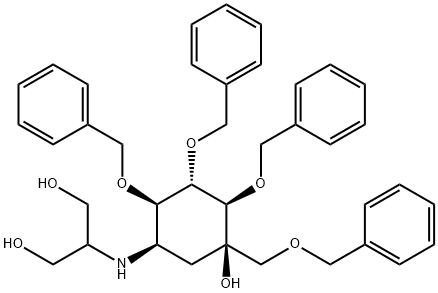
Manufacturing Process
N-(1,3-Dihydroxy-2-propyl)valiolamine:
To a solution of 2.0 g of valiol amine in 50 ml of N,N-dimethylformamide are
added 3.4 g of dihydroxyacetone, 1.5 ml of 2 N hydrochloric acid and 2.6 g of
sodium cyanoborohydride, followed by stirring at 60 to 70°C for 16 hours. The
reaction solution is concentrated under reduced pressure to distill off the N,N-
dimethylformamide as much as possible, and the residue is dissolved in 100 ml of water. The solution is made acid with 2 N hydrochloric acid, stirred for
30 to 40 minutes under ice-cooling, adjusted to pH 4.5 with 1 N sodium
hydroxide solution and subjected to column chromatography (250 ml) on
Dowex 50W x 8 (H + type) (produced by Dow Chemical of the United States of
America). After the washing with water, the elution is carried out with 0.5 N
aqueous ammonia. The eluate is concentrated under reduced pressure, and
the concentrate is chromatographed on a column (250 ml) of Amberlite CG-50
(NH 4 + type) (produced by Rohm and Haas Co. of the United States of
America), followed by the elution with water. The eluate is concentrated under
reduced pressure, and the concentrate is lyophilized to give 2.0 g of white
powder of N-(1,3-dihydroxy-2-propyl)valiol amine.
Ethanol (about 60 ml) is added to the above lyophilized product (1.2 g) of N-
(1,3-dihydroxy-2-propyl)valiol amine, and the mixture is warmed for 30
minutes in a hot water bath (the bath temperature: 90-95°C), followed by
leaving on standing in a refiegerator. The resultant crystalline substance is
recovered by filtration, washed with ethanol and then dried in a desiccator
under reduced pressure. Yield of 0.95 g. MP: 162-163°C. [α] D 25 = +26.2° (c
= 1, H 2 O).
brand name
Basen (Takeda); Glustat (Takeda).
Therapeutic Function
Antidiabetic, Antiobesity
World Health Organization (WHO)
Acarbose and voglibose area-glucosidase inhibitors and delay digestion/absorption of carbohydrates as well as improving postprandial hyperglycaemia.
General Description
The most potent -glucosidase inhibitor is the valiolamine derivative voglibose. Compared with acarbose its inhibitory potency in vitro, depending on the -glucosidase enzyme, is up to 200 times higher. In Japan, daily dosages are only 0.3 – 0.9 mg, however, therapeutic efficacy on HbA1c reduction could not be clearly demonstrated with those dosages. HbA1c, a glycated hemoglobin species, which occurs in blood, is a diagnostic measure for the non-enzymatic glycation process, and depends on the plasma glucose concentration. The process of protein glycation is discussed to be responsible for the development of diabetic complications (neuropathy, retinopathy, nephropathy). In Europe voglibose is under evaluation with distinctly higher daily dosages of 1.5 to 6 mg. In contrast to acarbose, the smaller inhibitors of the monosaccharide type (miglitol, emiglitate, voglibose) do not inhibit pancreatic -amylase.
Biological Activity
Orally active α -glucosidase inhibitor (IC 50 values are 3.9 and 6.4 nM at sucrase and maltase respectively). Increases glucagon-like peptide 1 (GLP-1) secretion and decreases food consumption in ob/ob mice, and reduces plasma concentrations of glucose, triglycerides and insulin in Wistar fatty rats. Exhibits antidiabetic and antiobesity activity in vivo .
Voglibose Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jinan Jianfeng Chemical Co., Ltd | 0531-88110457; +8615562555968 | info@pharmachemm.com | China | 241 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 885 | 50 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Shanghai finete Pharmaceutical Co., Ltd. | +86-18221039705 | CHINA | 139 | 55 | |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
Related articles
- Voglibose: Overview, Properties and Synthesis from Valiolamine
- Voglibose is a potent α-glucosidase inhibitor effective against hyperglycemia, obesity, and diabetes, with fewer side effects ....
- Apr 1,2024
- Voglibose: mechanism of action, pharmacokinetics and safety
- Voglibose is a α-glucosidase inhibitor for type 2 diabetes that can regulate blood glucose without causing hypoglycemia and ma....
- Aug 25,2023
- Role of voglibose in prevention of type 2 diabetes
- Voglibose (INN and USAN, trade name Voglib, marketed by Mascot Health Series) is an alpha-glucosidase inhibitor used for lower....
- Aug 19,2019
View Lastest Price from Voglibose manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Voglibose
83480-29-9
|
US $10.00 / kg | 1kg | 99.8% | 10000ton | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2023-09-06 | Voglibose
83480-29-9
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-06-27 | Voglibose
83480-29-9
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
83480-29-9(Voglibose)Related Search:
1of4