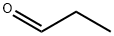Propionaldehyde
- CAS No.
- 123-38-6
- Chemical Name:
- Propionaldehyde
- Synonyms
- PROPANAL;1-Propanone;PROPIONIC ALDEHYDE;1-PROPANAL;C2H5CHO;Propional;N-PROPANAL;Propaldehyde;Propanaldehyde;Propin
- CBNumber:
- CB4852968
- Molecular Formula:
- C3H6O
Lewis structure
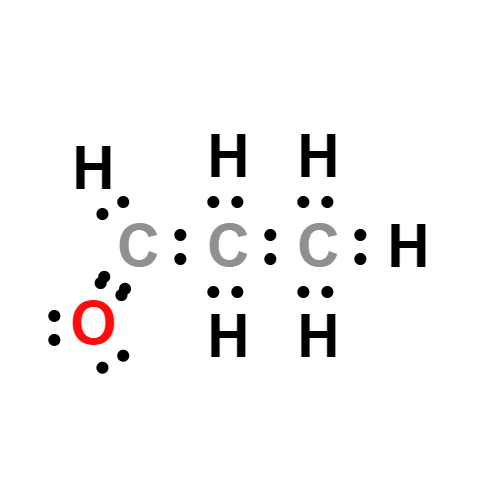
- Molecular Weight:
- 58.08
- MDL Number:
- MFCD00007020
- MOL File:
- 123-38-6.mol
| Melting point | -81 °C (lit.) |
|---|---|
| Boiling point | 46-50 °C (lit.) |
| Density | 0.805 g/mL at 25 °C (lit.) |
| vapor density | 2 (vs air) |
| vapor pressure | 18.77 psi ( 55 °C) |
| refractive index |
n |
| FEMA | 2923 | PROPIONALDEHYDE |
| Flash point | −16 °F |
| storage temp. | Store at <= 20°C. |
| solubility | soluble in chloroform; miscible with alcohol and ether |
| form | Liquid |
| Specific Gravity | 0.815 (20/4℃) |
| color | White |
| Odor | at 0.10 % in propylene glycol. earthy alcohol wine whiskey cocoa nutty |
| Odor Type | ethereal |
| Viscosity | 0.3167 cP (at 26.7°C) |
| explosive limit | 2.3-21%(V) |
| Odor Threshold | 0.001ppm |
| Water Solubility | 540 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| JECFA Number | 83 |
| Merck | 14,7823 |
| BRN | 506010 |
| Dielectric constant | 18.9(17℃) |
| Exposure limits | ACGIH: TWA 20 ppm |
| Stability | Stable. Highly flammable. Incompatible with oxidizing agents, strong acids, strong bases. |
| InChIKey | NBBJYMSMWIIQGU-UHFFFAOYSA-N |
| LogP | 0.59 at 25℃ |
| Substances Added to Food (formerly EAFUS) | PROPIONALDEHYDE |
| FDA 21 CFR | 172.515 |
| CAS DataBase Reference | 123-38-6(CAS DataBase Reference) |
| EWG's Food Scores | 3 |
| FDA UNII | AMJ2B4M67V |
| NIST Chemistry Reference | Propanal(123-38-6) |
| EPA Substance Registry System | Propionaldehyde (123-38-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302+H332-H315-H318-H335 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P303+P361+P353-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xi,Xn | |||||||||
| Risk Statements | 11-36/37/38-R36/37/38-R11-41-37/38-20/22 | |||||||||
| Safety Statements | 9-16-29-S9-S29-S16 | |||||||||
| RIDADR | UN 1275 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | UE0350000 | |||||||||
| Autoignition Temperature | 404 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29121900 | |||||||||
| Toxicity | LD50 orally in rats: 1.4 g/kg; LC for rats in air: 8000 ppm (Smyth) | |||||||||
| NFPA 704 |
|
Propionaldehyde price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W292312 | Propionaldehyde natural, ≥98%, FG | 123-38-6 | 100g | $99.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292312 | Propionaldehyde natural, ≥98%, FG | 123-38-6 | 1kg | $728 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292312 | Propionaldehyde natural, ≥98%, FG | 123-38-6 | 5kg | $3110 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292303 | Propionaldehyde ≥97%, FG | 123-38-6 | 1kg | $109 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292303 | Propionaldehyde ≥97%, FG | 123-38-6 | 4kg | $196 | 2024-03-01 | Buy |
Propionaldehyde Chemical Properties,Uses,Production
Description
Propionaldehyde is a volatile liquid substance which consists of one carbonyl group and its characteristic functional group. The main functional group is an aldehyde which classifies propionaldehyde in the carbonyls. The main trunk of this substance is a short aliphatic chain. The carbonyl group determines, to a large extent, its chemical properties and most importantly its nucleophilic property. Propionaldehyde is readily oxidized if in contact to oxygen and should therefore be stored under inert gases.
Chemical Properties
Propionaldehyde has a characteristic sharp and pungent odor similar to acetaldehyde.It undergoes reactions typical for the low molecular weight aldehydes, which, because of the terminal carbonyl group, are very reactive. Contamination or the exposure to elevated temperatures may induce a hazardous polymerization.

Propionaldehyde is a colorless, flammable liquid with a suffocating fruity odor. It is used as Intermediate for the chemical industry, for example for the manufacture of pharmaceuticals, pesticides, perfumes and plastics.
Occurrence
Reported found in apple aroma and in the essential oils of camphor, Rosa centifolia, clary sage, Pinus excelsa and Pinus silverstris. Also reported found in over 100 natural products including apple, banana, sweet cherry, sour cherry, black currant grapes, melon, pineapple, strawberry, cabbage, carrot, celery, cucumber, garlic, onion, leek, potato, peas, rutabaga, tomato, Scotch spearmint oil, vinegar, bread and bread preferment, blue cheeses, cheddar cheese, Swiss cheese, butter, milk, cream, boiled egg, caviar, fatty fish, cooked turkey, beef, pork and chicken, beer, rum, cognac, whiskies, grape wines, coffee, cocoa, tea, roasted filberts and peanuts, peanut oil, potato chips, pecans, oats, honey, soybeans, Arctic bramble, beans, Bantu beer, plum brandy, cauliflower, Brussels sprouts, rice, prickly pear, peated malt, clary sage, truffle, krill, oysters, loganberry, Chinese quince and maté.
Uses
Propionaldehyde is produced by the oxo reaction of ethylene with carbon monoxide and hydrogen. n-Propyl alcohol is produced by hydrogenation of propionaldehyde, and propionic acid is made by oxidation of propionaldehyde.
n-Propyl alcohol is used as solvent in printing inks and as an intermediate in the preparation of agricultural chemicals. Propionic acid is used as a grain preservative as, for example, in preventing spoilage of wet corn used as animal feed. The use of propionic acid as a grain preservative is an alterna tive to drying by heating, which consumes fuel, and is considered mostly when fuel is expensive.
Uses
Manufacture of propionic acid, polyvinyl, and other plastics; synthesis of rubber chemicals; disin- fectant; preservative.
Uses
Propionaldehyde is used in the productionof propionic acid, propionic anhydride, andmany other compounds. It is formed in theoxidative deterioration of corn products, suchas corn chips. It occurs in automobile exhaustgases.
Preparation
By oxidation of propyl alcohol, or by dry distillation of barium propionate with calcium formate.
Preparation
Propionaldehyde is prepared in the usual way by the oxidation of n-propyl alcohol. It can also be prepared by dehydrogenating the alcohol by passing the vapors over a heated copper or brass catalyst. This avoids the danger of further oxidation to propionic acid.
The reactions of propionaldehyde are practically like those of acetaldehyde. It must be remembered that only the two alpha hydrogen atoms are active in replacement and condensation reactions.
Definition
ChEBI: An aldehyde that consists of ethane bearing a formyl substituent. The parent of the class of propanals.
General Description
A clear colorless liquid with an overpowering fruity-like odor. Less dense than water. Flash point 15°F. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
Propionaldehyde may form explosive peroxides. Reacts vigorously with oxidizing agents. Explosive in the form of vapor when exposed to heat or flame [Lewis]. Incompatible with strong bases and strong reducing agents. Vigorous polymerization reaction with methyl methacrylate. Polymerization may also occur in the presence of acids or caustics .
Hazard
Flammable, dangerous fire risk, explosive limits in air 3.0–16%. Upper respiratory tract irri- tant.
Health Hazard
Propionaldehyde is a mild irritant to humanskin and eyes. The irritation effect from40 mg was severe in rabbits’ eyes. The toxicityof this compound observed in test animalswas low. Subcutaneous administration in ratsexhibited the symptoms of general anestheticeffect, convulsion, and seizure. Inhalationtoxicity was determined to be low. A concentrationof 8000 ppm (19,000 mg/m3) inair was lethal to rats.
LD50 value, oral (rats): 1400 mg/kg
LD50 subcutaneous (rats): 820 mg/kg.
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel considerable distance to a source of ignition and flash back.
Flammability and Explosibility
Flammable
Biochem/physiol Actions
Taste at 5-10 ppm
Safety Profile
Moderately toxic by skin contact, ingestion, and subcutaneous routes. Mddly toxic by inhalation. A skin and severe eye irritant. Flammable liquid. Dangerous fire hazard when exposed to heat or flame; reacts vigorously with oxidizers. Explosive in the form of vapor when exposed to heat or flame. Vigorous polymerization reaction with methyl methacrylate. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES.
Potential Exposure
Used as a synthetic flavoring; as a disinfectant and preservative; to make propionic acid; in plastic and rubber manufacturing; to make alkyl resins and plasticizers.
Carcinogenicity
In a mutagenic test in V79 cells, Eder et al. observed that propionaldehyde is not mutagenic at 1 mM, but is toxic at 2mM. Similar to acrolein, Eder et al. suggested that the mutagenicity of this compound is mediated by its bifunctionality, whereas its cytotoxicity is mediated by the aldehyde function.
Shipping
UN1275 Propionaldehyde, Hazard Class: 3; Labels: 3-Flammable liquid.
Propionaldehyde is readily oxidized if in contact to oxygen and should therefore be stored under inert gases.
Purification Methods
the aldehyde with CaSO4 or CaCl2, and fractionally distil it under nitrogen or in the presence of a trace of hydroquinone (to retard oxidation). Blacet and Pitts [J Am Chem Soc 74 3382 1952] repeatedly distilled the middle fraction in a vacuum until it no longer gave a solid polymer when cooled to -80o. It is stored with CaSO4. [Beilstein 1 IV 3165.]
Incompatibilities
Incompatible with strong acids; amines. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoa- cids, epoxides. Strong caustics; reducing agents can cause explosive polymerization. Can self-ignite if finely dispersed on porous or combustible material, such as fabric. Heat or UV light can cause decomposition. Aldehydes are fre- quently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are gener- ated by the combination of aldehydes with azo, diazo com- pounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (anti- oxidants) to shipments of aldehydes retards autoxidation
Waste Disposal
Propionaldehyde is destroyed by burning in achemical incinerator equipped with an afterburnerand scrubber. Permanganate oxidationis a suitable laboratory method of destruction.
Propionaldehyde Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of5
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2966 | 58 |
| QUALITY CONTROL CHEMICALS INC. | (323) 306-3136 | orders@qcchemical.com | United States | 26 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 | overseasales1@yongstandards.com | United States | 14336 | 58 |
View Lastest Price from Propionaldehyde manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-17 | Propionaldehyde
123-38-6
|
US $1.00 / KG | 1KG | ≥99% | 1000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2023-09-23 | Propionaldehyde
123-38-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-07-27 | Propionaldehyde
123-38-6
|
US $1.10 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd |
-

- Propionaldehyde
123-38-6
- US $1.00 / KG
- ≥99%
- Jinan Finer Chemical Co., Ltd
-

- Propionaldehyde
123-38-6
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Propionaldehyde
123-38-6
- US $1.10 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd
123-38-6(Propionaldehyde)Related Search:
1of4