Selenious acid
- CAS No.
- 7783-00-8
- Chemical Name:
- Selenious acid
- Synonyms
- SELENOUS ACID;SELENIUM METAL;selenious;Selenius Acid;SELENIUM STANDARD;YEAST BOUND SELENIUM;YXS;VANDEX;selaniuM;SELENIUM RED
- CBNumber:
- CB4854326
- Molecular Formula:
- H2O3Se
Lewis structure
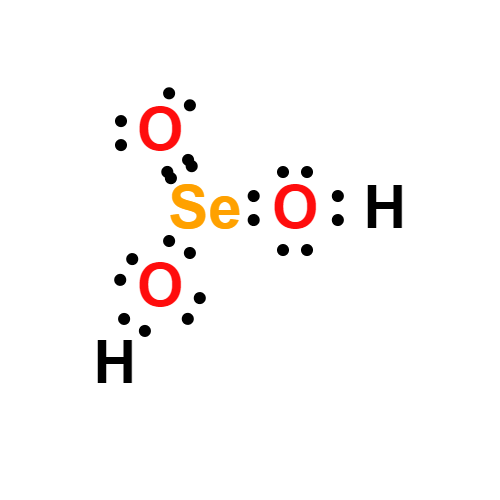
- Molecular Weight:
- 128.97
- MDL Number:
- MFCD00134090
- MOL File:
- 7783-00-8.mol
- MSDS File:
- SDS
| Melting point | 70 °C (dec.) (lit.) |
|---|---|
| Boiling point | 684.9 °C(lit.) |
| Density | 3.004 g/mL at 25 °C (lit.) |
| vapor pressure | 2.7hPa at 15℃ |
| Flash point | 690°C |
| storage temp. | Store at RT. |
| solubility | H2O: soluble |
| pka | 2.46(at 25℃) |
| form | powder |
| color | White to almost white or light pink |
| Specific Gravity | 3.004 |
| PH | 3.15(1 mM solution);2.47(10 mM solution);1.9(100 mM solution) |
| Water Solubility | 167 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8430 |
| Exposure limits |
ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3 |
| Stability | Stable. |
| CAS DataBase Reference | 7783-00-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | F6A27P4Q4R |
| EPA Substance Registry System | Selenious acid (H2SeO3) (7783-00-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H373-H410 | |||||||||
| Precautionary statements | P273-P301+P310+P330-P304+P340+P311-P314 | |||||||||
| Hazard Codes | T,N,Xi | |||||||||
| Risk Statements | 36/38-50/53-33-23/25-52/53 | |||||||||
| Safety Statements | 61-60-45-28A-20/21-26-28 | |||||||||
| RIDADR | UN 3440 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | VS7700000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28111980 | |||||||||
| NFPA 704 |
|
Selenious acid Chemical Properties,Uses,Production
Description
Selenious acid is a colourless, deliquescent crystal in appearance. It decomposes on heating,
producing water and toxic fumes of selenium oxides.
It is incompatible with strong reducing agents, organic materials, and finely powdered
metals and reacts on contact with acids producing toxic gaseous hydrogen selenide.
Selenious acid is a non-combustible chemical. On fire/burning, it emits irritating or toxic
fumes (or gases).
The major use of selenious acid is in changing the colour of steel, especially the steel in
guns from silver grey to blue grey. It is also used for the chemical darkening and patination
of copper brass and bronze, producing a rich dark-brown colour that can be further
enhanced with mechanical abrasion.
Chemical Properties
Selenious acid is a colorless, crystalline solid.
Chemical Properties
White solid
Uses
As an oxidizing agent, as a reagent for alkaloidsSelenous acid is used in steel guns, wherein it changes the color of the steel from silver-grey to blue-grey. It serves as an important component of the Mecke reagent, which is utilized for drug testing. It acts as a source of selenium. It acts as a reagent for alkaloids and an oxidizing agent. Further, it is used in labeling radiopharmaceuticals. It is involved in the organic synthesis for the preparation of glyoxal.
Uses
As a reagent for alkaloids; as oxidizing agent.
Definition
ChEBI: Selenous acid is a selenium oxoacid. It is a conjugate acid of a hydrogenselenite.
General Description
Colorless solid, transparent, colorless crystals. Used as a reagent for alkaloids and as an oxidizing agent. Isotope is used in labeling radiopharmaceuticals.
Reactivity Profile
Selenious acid decomposes when heated to toxic and volatile selenium dioxide. Serves as an oxidizing agent. Reacts exothermically with many reducing agents including hydroiodic acid, sulfurous acid, sodium hyposulfite, hydroxylamine salts, hydrazine salts, hypophosphorous acid, phosphorous acid [Merck]. Oxidizes many organic substances. Is oxidized to selenic acid by strong oxidizing agents.
Hazard
Toxic by inhalation, ingestion, and skin absorption.
Health Hazard
Selenious acid and its salts are capable of penetrating the skin and can produce acute poisonings. Causes irritations and burns of the skin. It is highly toxic orally. Inorganic selenium compounds may cause dermatitis.
Fire Hazard
When heated to decomposition Selenious acid emits toxic fumes of selenium. Avoid heating.
Safety Profile
Poison by ingestion, intraperitoneal, and intravenous routes. Human mutation data reported. Used as an oxidizing agent. When heated to decomposition it emits toxic fumes of Se. See also SELENIUM COMPOUNDS
Potential Exposure
Selenious acid is used as a reagent for alkaloids and as an oxidizing agent. Isotope is used in labeling radiopharmaceuticals.
Shipping
UN3283Selenium compound, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous material, Technical Name Required. UN3440 Selenium compound, liquid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous material, Technical Name Required.
Purification Methods
Recrystallise the acid from water. On heating it loses water and SeO2 sublimes. [Waitkins & Clark Chem Rev 36 235 1945.]
Incompatibilities
A strong oxidizer. Reacts exothermically with many reducing agents including hydroiodic acid, sulfurous acid, sodium hyposulfite, hydroxylamine salts, hydrazine salts, hypophosphorous acid, phosphorous acid. Incompatible combustibles, organic material, oxidizable materials, strong acids, strong bases. Contact with acids produce toxic and gaseous hydrogen selenide. Attacks metals.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal.





