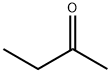
2-Butanone
- CAS No.
- 78-93-3
- Chemical Name:
- 2-Butanone
- Synonyms
- MEK;METHYL ETHYL KETONE;BUTANONE;Butan-2-one;ETHYL METHYL KETONE;2-Butanone,99%;2-Butanon;METHYL ETHYL KETONE (MEK)(BUTANONE);Butanon;MEK = 2-BUTANONE
- CBNumber:
- CB4854386
- Molecular Formula:
- C4H8O
Lewis structure
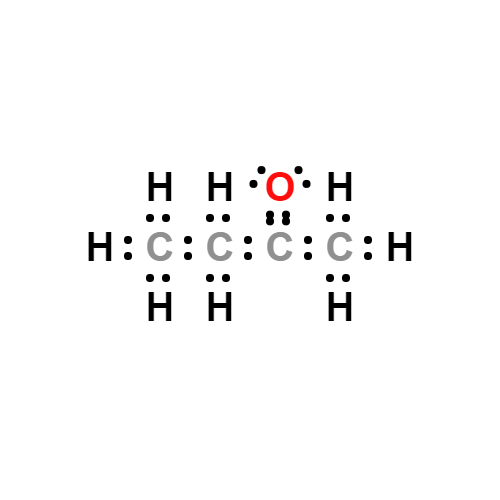
- Molecular Weight:
- 72.11
- MDL Number:
- MFCD00011648
- MOL File:
- 78-93-3.mol
- MSDS File:
- SDS
| Melting point | -87 °C (lit.) |
|---|---|
| Boiling point | 80 °C (lit.) |
| Density | 0.805 g/mL at 25 °C (lit.) |
| vapor density | 2.49 (vs air) |
| vapor pressure | 71 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 2170 | 2-BUTANONE |
| Flash point | 26 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with acetone, ethanol, benzene, ether (U.S. EPA, 1985), and many other solvents, particularly ketones and aldehydes |
| pka | 14.7 (quoted, Riddick et al., 1986) |
| form | Solution |
| color | Colorless |
| Relative polarity | 0.327 |
| Odor | Sweet/sharp odor detectable at 2 to 85 ppm (mean = 16 ppm) |
| PH | pH(1+4, 25℃):6.0~7.0 |
| explosive limit | 1.8-11.5%(V) |
| Odor Threshold | 0.44ppm |
| Odor Type | ethereal |
| Water Solubility | 290 g/L (20 ºC) |
| Merck | 14,6072 |
| JECFA Number | 278 |
| BRN | 741880 |
| Henry's Law Constant | 23.0 at 50.00 °C, 34.1 at 60.00 °C, 50.6 at 70.00 °C, 70.4 at 80.00 °C (headspace-GC, Hovorka et al., 2002) |
| Exposure limits | TLV-TWA, PEL 590 mg/m3 (200 ppm) (ACGIH, OSHA); STEL 885 mg/m3 (300 ppm) (ACGIH); IDLH 3000 ppm (NIOSH). |
| Dielectric constant | 18.5(20℃) |
| Stability | Stable. Highly flammable. Incompatible with oxidizing agents, bases, strong reducing agents. Protect from moisture. |
| LogP | 0.3 at 40℃ |
| Substances Added to Food (formerly EAFUS) | 2-BUTANONE |
| FDA 21 CFR | 172.515; 175.105; 175.320; 177.1200 |
| CAS DataBase Reference | 78-93-3(CAS DataBase Reference) |
| EWG's Food Scores | 3 |
| FDA UNII | 6PT9KLV9IO |
| NIST Chemistry Reference | 2-Butanone(78-93-3) |
| EPA Substance Registry System | Methyl ethyl ketone (78-93-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H319-H336 | |||||||||
| Precautionary statements | P210-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xi,T | |||||||||
| Risk Statements | 11-36-66-67-39/23/24/25-23/24/25 | |||||||||
| Safety Statements | 9-16-45-36/37 | |||||||||
| RIDADR | UN 1193 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | EL6475000 | |||||||||
| Autoignition Temperature | 516 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29141200 | |||||||||
| Toxicity | LD50 orally in rats: 6.86 ml/kg (Smyth) | |||||||||
| IDLA | 3,000 ppm | |||||||||
| NFPA 704 |
|
2-Butanone Chemical Properties,Uses,Production
Description
2-Butanone is a stable, highly flammable chemical. It is incompatible with oxidising agents, bases, and strong reducing agents. It is a colourless liquid with a sharp, sweet odour. 2-Butanone is produced in large quantities. It is used as a solvent and nearly half of its use is in paints and other coatings because it will quickly evaporate into the air and it dissolves many substances. It is also used in glues and as a cleaning agent.
Chemical Properties
Methyl ethyl ketone (MEK) is a colorless liquid with an odor that has been described as moderately sharp, fragrant, peppermint, or acetone like. It soluble in water up to 28% by weight and is miscible with many other organic solvents. The lower explosive limit is 1.4% and the upper explosive limit is 11.4%. Methyl ethyl ketone may be incompatible with strong oxidizers, amines, ammonia, inorganic acids, caustics, isocyanates, and pyridines. When used industrially, methyl ethyl ketone must be handled with caution, as it is a Class lB flammable liquid NIOSH (2010).
Physical properties
2-Butanone is a clear, colorless, volatile, very flammable liquid with a sweet, mint or acetone-like odor. Odor threshold concentration is 10.0 ppmv (Leonardos et al., 1969). Experimentally determined detection and recognition odor threshold concentrations were 5.8 mg/m3 (2.0 ppmv) and 16 mg/m3 (5.4 ppmv), respectively (Hellman and Small, 1974). It is incompatible with oxidizing agents, bases, and strong reducing agents. It is a colorless liquid with a sharp, sweet odor. 2-Butanone is produced in large quantities. It is used as a solvent and nearly half of its use is in paints and other coatings because it quickly evaporates into the air and it dissolves many substances. It is also used in glues and as a cleaning agent.
Occurrence
Reported found as an impurity among products from the dry distillation of wood and in the oil (extracted with ether) of black tea; it is also present in coffee, cheese, bread, some citrus oils and some other natural products (grape, raspberry).
Uses
MEK is used as a solvent for various coating systems, for
example, vinyl, adhesives, nitrocellulose, and acrylic coatings.
It is used in paint removers, lacquers, varnishes, spray paints,
sealers, glues, magnetic tapes, printing inks, resins, rosins,
cleaning solutions, and for polymerization. It is found in other
consumer products, for example, household and hobby
cements, and wood-filling products. MEK is used in dewaxing
lubricating oils, the degreasing of metals, in the production of
synthetic leathers, transparent paper and aluminum foil, and as
a chemical intermediate and catalyst. It is an extraction solvent
in the processing of foodstuffs and food ingredients. MEK can
also be used to sterilize surgical and dental equipment.
In addition to its manufacture, environmental sources of
MEK include exhaust from jet and internal combustion
engines, and industrial activities such as gasification of coal. It
is found in substantial amounts in tobacco smoke. MEK is
produced biologically and has been identified as a product of
microbial metabolism. It has also been found in plants, insect
pheromones, and animal tissues, and MEK is probably a minor
product of normal mammalian metabolism. It is stable under
ordinary conditions but can form peroxides on prolonged
storage; these may be explosive.
Uses
Methyl ethyl ketone (2-butanone, ethyl methyl ketone, methyl acetone) is an organic solvent of relatively low toxicity, which is found in many applications. It is used in industrial and commercial products as a solvent for adhesives, paints, and cleaning agents and as a de-waxing solvent. A natural component of some foods, methyl ethyl ketone can be released into the environment by volcanoes and forest fires.It is used in themanufacture of smokeless powder and colorless synthetic resins, as a solvent, and insurface coating. It is also used as a flavoringsubstance in food.
Uses
2-Butanone is an eye irritant that has been used as a water soluble photoinitiator for the photopolymerization of methacrylic acid (MAA). As solvent; in the surface coating industry; manufacture of smokeless powder; colorless synthetic resins.
Definition
ChEBI: A dialkyl ketone that is a four-carbon ketone carrying a single keto- group at position C-2.
Preparation
By catalytic dehydrogenation of secondary butyl alcohol; by dehydration of butane-2,3-diol by refluxing with 25% aqueous H2SO4. Industrially, it is also prepared by controlled oxidation of butane, by dry distillation of calcium acetate and calcium propionate, or by refluxing methyl acetoacetate and diluted H2SO4.
Definition
A colorless volatile liquid ketone. It is manufactured by the oxidation of butane and used as a solvent.
Production Methods
Methyl ethyl ketone is commercially manufactured from nbutene in a metal-catalyzed hydrogenation reaction that proceeds through the intermediate formation of 2-butanol . A second method of synthesis involves the liquid- phase oxidation of n-butane with the formation of acetic acid as a coproduct.
Taste threshold values
Taste characteristics at 5 ppm: chemical-like and slightly fruity green.
General Description
Methyl ethyl ketone (MEK) is a colourless liquid with a sweet and sharp odour. It is soluble in alcohol, ether, acetone benzene, and water. It is a solvent often found in mixtures with acetone, ethyl acetate, n-hexane, toluene, or alcohols. It has applications in the surface coating industry and in the de-waxing of lubricating oils. MEK is used in the manufacture of colourless synthetic resins, artificial leather, rubbers, lacquers, varnishes, and glues.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Amines are chemical bases. They neutralize acids to form salts plus water.These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides.They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Hazard
Flammable, dangerous fire risk. Toxic by ingestion.
Health Hazard
Occupational workers are exposed to 2-butanone by breathing contaminated air in workplaces associated with the production or use of paints, glues, coatings, or cleaning agents. Prolonged exposures to 2-butanone cause symptoms of poisoning such as cough, dizziness, drowsiness, headache, nausea, vomiting, dermatitis, irritation of the nose, throat, skin, and eyes and at very high levels cause drooping eyelids, uncoordinated muscle movements, loss of consciousness, and birth defects. Chronic inhalation studies in animals have reported slight neurological, liver, kidney, and respiratory effects. However, information on the chronic (long-term) effects of 2-butanone (methyl ethyl ketone) in humans is limited.
Health Hazard
The acute toxicity of methyl ethyl ketone is low. Exposure to high concentrations
can cause headache, dizziness, drowsiness, vomiting, and numbness of the
extremities. Irritation of the eyes, nose, and throat can also occur. Methyl ethyl
ketone is considered to have adequate warning properties.
Repeated or prolonged skin exposure to methyl ethyl ketone can cause defatting of
the skin, leading to cracking, secondary infection, and dermatitis. This compound
has not been found to be carcinogenic or to show reproductive or developmental
toxicity in humans. Methyl ethyl ketone has exhibited developmental toxicity in
some animal tests
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Methyl ethyl ketone is extremely flammable (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." MEK vapor forms explosive mixtures with air at concentrations of 1.9 to 11% (by volume). Carbon dioxide or dry chemical extinguishers should be used for MEK fires.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Pharmacology
Anticonvulsant activity was demonstrated in rats given orally a dose of 805 mg MEK/kg, which significantly delayed the onset of isonicotinic acid hydrazide-induced convulsions and provided 60% protection against electroshock convulsions, but failed to protect against metrazole convulsions (Kohli, Kishor, Dua & Saxena, 1967). Golubev (1969) reported that 0.25 M-MEK caused contraction of the rabbit pupil, and MEK and other volatile substances isolated from human urine and injected iv into rabbits damaged cerebral and coronary arteries and caused increased capillary permeability, but did not alter the blood-sugar level (Mabuchi, 1969). In dogs, MEK caused vomiting, muscular debility and the formation of large quantities of urinary magnesium ammonium phosphate crystals (Verstraete, van der Stock & Mattheeuws, 1964).
Combined ip administration of MBK and MEK (1:3) to guinea-pigs increased the urinary excretion of the MBK metabolites 2-hexanol and 2,5-hexanedione (Couri, Abdel-Rahman & Hetland, 1976). If the neurotoxic action of MBK is mediated by a metabolite, stimulation of MBK metabolism by simultaneous exposure to MEK may help to explain the marked enhancement of neurotoxicity that is observed with combined MBK/MEK exposures (Hetland et al. 1976).
Safety Profile
Moderately toxic by ingestion, skin contact, and intraperitoneal routes. Human systemic effects by inhalation: conjunctiva irritation and unspecified effects on the nose and respiratory system. An experimental teratogen. A strong irritant. Human eye irritation @ 350 ppm. Affects peripheral nervous system and central nervous system. Highly flammable liquid. Reaction with hydrogen peroxide + nitric acid forms a heatand shock-sensitive explosive product. Ignition on contact with potassium tert-butoxide. Mixture with 2- propanol will produce explosive peroxides during storage. Vigorous reaction with chloroform + alkali. Incompatible with chlorosulfonic acid, oleum. To fight fire, use alcohol foam, CO2, dry chemical. Used in production of drugs of abuse. When heated to decomposition it emits acrid smoke and fumes.
Potential Exposure
MEK is used as a solvent in nitrocellulose coating and vinyl film manufacture; in smokeless powder manufacture; in cements and adhesives and in the dewaxing of lubricating oils. It is also an intermediate in drug manufacture
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Carcinogenicity
Although MEK has not been specifically examined in a rodent 2-year bioassay, there is little to suggest that the material is carcinogenic. When used as a delivery vehicle in a dermal carcinogenicity bioassay for organic sulfur compounds, Horton et al. found that the application of benzyl disulfide or phenylbenzylthiophene in a 25–29% solution of MEK in dodecylbenzene together failed to increase the incidence of benign skin papillomas in male C3H/HeJmice. The mice used in the experiments were treated twice a week for 52 weeks with the MEKcontaining test solution.
Source
Improper disposal of cleaning fluids, adhesives, paints, and lacquers, and laboratory
solvent. Leaches from PVC cement used to join tubing (Wang and Bricker, 1979). Also present in
cigarette smoke (500 ppm) and exhaust from gasoline-powered engines (<0.1–2.6 ppm)
(Verschueren, 1983).
Gas-phase tailpipe emission rates from California Phase II reformulated gasoline-powered
automobiles with and without catalytic converters were 0.47 and 32 mg/km, respectively (Schauer
et al., 2002).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rates of 2-butanone were 215 mg/kg of pine burned, 115 mg/kg of oak burned, and 77 mg/kg of
eucalyptus burned.
Environmental Fate
Biological. Following a lag time of approximately 5 h, 2-butanone degraded in activated sludge
(30 mg/L) at a rate constant ranging from 0.021 to 0.025/h (Urano and Kato, 1986).
Bridié et al. (1979) reported BOD and COD values of 2.03 and 2.31 g/g using filtered effluent
from a biological sanitary waste treatment plant. These values were determined using a standard
dilution method at 20 °C for a period of 5 d. The ThOD for 2-butanone is 2.44 g/g. Using the BOD
technique to measure biodegradation, the mean 5-d BOD value (mM BOD/mM 2-butanone) and
ThOD were 3.23 and 58.7%, respectively (Vaishnav et al., 1987).
Photolytic. Synthetic air containing gaseous nitrous acid and exposed to artificial sunlight (λ =
300–450 nm) photooxidized 2-butanone into peroxyacetyl nitrate and methyl nitrate (Cox et al.,
1980). They reported a rate constant of 2.6 x 10-12 cm3/molecule?sec for the reaction of gaseous 2-
butane with OH radicals based on a value of 8 x 10-12 cm3/molecule?sec for the reaction of
ethylene with OH radicals.
The OH radical-initiated photooxidation of 2-butanone in a smog chamber produced
peroxyacetyl nitrate and acetaldehyde (Cox et al., 1981). Reported rate constants for the reaction
of 2-butanone with OH radicals in the atmosphere and in water are 1.15 x 10-13 and 1.50 x 10-13
cm3/molecule?sec, respectively (Wallington and Kurylo, 1987; Wallington et al., 1988a). The rate
constant for the reaction of 2-butanone and OH radicals in the atmosphere at 300 K is 2.0 x 10-12
cm3/molecule?sec (Hendry and Kenley, 1979). Cox et al. (1981) reported a photooxidation half-life
of 2.3 d for the reaction of 2-butanone and OH radicals in the atmosphere.
Chemical/Physical. 2-Butanone will not hydrolyze because it has no hydrolyzable functional
group (Kollig, 1993).
Combustion in air will produce carbon monoxide (incomplete combustion), carbon dioxide, and
water vapor.
At an influent concentration of 1.0 g/L, treatment with GAC resulted in an effluent
concentration of 532 mg/L. The adsorbability of the carbon used was 94 mg/g carbon (Guisti et
al., 1974).
storage
2-Butanone should be protected from moisture.
Shipping
UN1193 Methyl ethyl ketone or Ethyl methyl ketone, Hazard Class: 3; Labels: 3-Flammable liquid.
Toxicity evaluation
There is very limited information on the mechanisms of toxicity of MEK. Relatively high-inhaled concentrations of 1475–29 500 mg m-3 (500–10 000 ppm) caused pulmonary vasoconstriction and hypertension in cats and dogs. There are several human case reports of neurological effects resulting from high exposure to MEK in combination with other solvents, and animal studies have confirmed synergism between MEK and ethyl n-butyl ketone, methyl n-butyl ketone, n-hexane, carbon tetrachloride, 2,5-hexanedione, and chloroform. The main target organs involved in toxicological interactions are the nervous system and liver, and the lung has also been mentioned.
Incompatibilities
May form explosive mixture with air. Violent reaction with strong oxidizers, amines, ammonia, inorganic acids; caustics, isocyanates, pyridines. Incompatible with potassium tert-butoxide, 2-propanol, chlorosulfonic acid; oleum. Attacks some plastics. Ketones are incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrated amines, azo, diazo, azido compounds, carbamates, organic cyanates
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration
Precautions
2-Butanone vapor and air mixtures are explosive. It reacts violently with strong oxidants and inorganic acids causing fi re and explosion hazard.
2-Butanone Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of8
78-93-3(2-Butanone)Related Search:
1of4