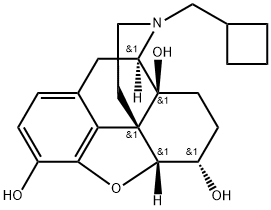
nalbuphine
- CAS No.
- 20594-83-6
- Chemical Name:
- nalbuphine
- Synonyms
- C07251;NALBUPNINE;Nalbuphinebase;2H3]-Nalbuphine;NaltrexoneImpurity24;NETZHAKZCGBWSS-CEDHKZHLSA-N;Nalbuphine (1.0mg/ml in Acetonitrile);Nalbuphine (base and/or unspecified salts);17-[Cyclobutylmethyl]-4,5α-epoxymorphinan-3,6α,14-triol;Morphinan-3,6,14-triol, 17-(cyclobutylmethyl)-4,5-epoxy-, (5α,6α)- (9CI)
- CBNumber:
- CB4918699
- Molecular Formula:
- C21H27NO4
- Molecular Weight:
- 357.45
- MDL Number:
- MOL File:
- 20594-83-6.mol
| Melting point | 230.5° |
|---|---|
| Boiling point | 566.6±50.0 °C(Predicted) |
| Density | 1.44±0.1 g/cm3(Predicted) |
| solubility | soluble in Methanol |
| form | Solid |
| pka | 9.39±0.60(Predicted) |
| color | White |
| FDA UNII | L2T84IQI2K |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
nalbuphine Chemical Properties,Uses,Production
Chemical Properties
Pale Yellow Solid
Originator
Nubain,Du Pont,US,1979
Uses
Mixed opioid agonist-antagonist. Analgesic (narcotic).
Uses
Nalbuphine is a mixed opioid agonist-antagonist. Nalbuphine is an analgesic (narcotic).
Definition
ChEBI: Nalbuphine is an organic heteropentacyclic compound. It has a role as a mu-opioid receptor antagonist and an opioid analgesic. It derives from a hydride of a morphinan.
Manufacturing Process
To a slurry of 110.5 g of 14-hydroxydihydronormorphinone in 2.5 liters of
methylene chloride and 280 ml of triethylamine was added a solution of 106 g
of cyclobutanecarboxylic acid chloride in 500 ml of methylene chloride. The
temperature of the reaction mixture was maintained at 20°C to 25°C during
the addition. After 5 minutes the reaction mixture was brought to reflux and
heated for 5 hours.
It was then cooled, washed with water, dried over sodium sulfate and
evaporated to dryness. The residue was crystallized from benzene and
pentane to give 138.5 g of the dicyclobutanecarbonyl derivative, melting point
about 112°C (dec.).
The dicyclobutanecarbonyl derivative (136.7 g) was dissolved in 200 ml of
tetrahydrofuran and added dropwise to a suspension of 34.2 g of lithium
aluminum hydride in 1 liters of tetrahydrofuran. The temperature of the
mixture rose to reflux during the addition. Reflux was maintained for 2 hours
after the addition was completed. After cooling, 110 ml of ethyl acetate was
added dropwise, followed by 30 ml of water, followed by a solution of 53 g of
ammonium chloride in 125 ml of water. The resulting mixture was filtered and
the inorganic precipitate was washed with methanol. Evaporation of the
combined filtrates gave 66 g of N-cyclobutylmethyl-14-hydroxydihydronormorphinone, melting point 229°C to 231°C.
brand name
Nubain (Endo).
Therapeutic Function
Analgesic
nalbuphine Preparation Products And Raw materials
Raw materials
1of2