dichlorine oxide
- CAS No.
- 7791-21-1
- Chemical Name:
- dichlorine oxide
- Synonyms
- Chloroxydul;dichlorine oxide;dichloridooxygen;Chlorine monoxide.;Dichlorine monooxide;Chlorine oxide (Cl2O)
- CBNumber:
- CB4926870
- Molecular Formula:
- Cl2O
Lewis structure
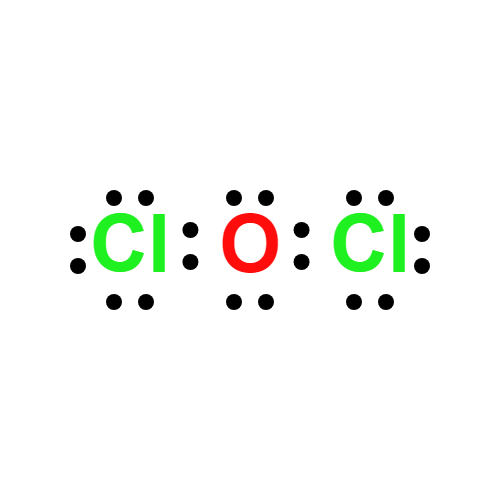
- Molecular Weight:
- 86.91
- MDL Number:
- MOL File:
- 7791-21-1.mol
| Melting point | -120.6° |
|---|---|
| Boiling point | bp 2.2° |
| Density | 1.549 (estimate) |
| solubility | very soluble in H2O |
| form | yellow-brown gas |
| color | yellow-brown |
| Water Solubility | 1 volume of water dissolves more than 100 volumes (0°C); saturation solubility is 143.6g/100g H2O (?9.4°C) [MER06] [KIR78] |
| FDA UNII | 0EQ5I4TK19 |
| EPA Substance Registry System | Chlorine monoxide (7791-21-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314-H400-H411-H290-H318 | |||||||||
| Precautionary statements | P234-P390-P404-P280-P305+P351+P338-P310-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P273-P391-P501 | |||||||||
| NFPA 704 |
|
dichlorine oxide Chemical Properties,Uses,Production
Reactions
The oxidation state of chlorine is +1. The compound is highly unstable, decomposing to chlorine and oxygen when exposed to light, heat, spark, or under catalytic conditions. It reacts with hot water forming hypochlorous acid:
Cl2O + H2O → 2HOCl
It oxidizes a number of compounds, undergoing violent decomposition. It reacts with metals under controlled conditions, forming their hypochlorites.
Chemical Properties
yellowish brown gas; disagreeable penetrating odor; explodes on contact with organic matter; decomposes at a moderate rate at room temp; anhydride of hypochlorous acid; Henry’s constant at 3.46°C is 14.23kPa/(molarity); enthalpy of vaporization 25.9kJ/mol; can be prepared by reacting Cl2 with HgO; used as an intermediate in manufacture of calcium hypochlorite and in sterilization; reacts with a variety of organic compounds [MER06] [KIR78]
Physical properties
Yellowish-brown gas; disagreeable suffocating odor; unstable at room temperature; gas density 3.89 g/L at 0°C; condenses to a reddish brown liquid at 2.2°C; freezes at -20°C; highly soluble in water; also soluble in alkalis, sulfuric acid, and carbon tetrachloride.
Uses
Dichlorine oxide is a chlorinating agent.
Uses
Chlorine monoxide is used as a strongand selective chlorinating agent. It is storedbelow -80°C ( -112°F) as a liquid or solid.
Preparation
Chlorine monoxide is prepared by passing chlorine gas over yellow mercuric oxide. It is stored below -80°C as a liquid or solid.
Definition
An orange gas made by passing chlorine over mercury(II) oxide. It is a strong oxidizing agent and dissolves in water to give chloric( I) acid.
General Description
Red-yellow gas. Very reactive and unstable. Unusually stored as hydrate in frozen form. Used as a wood bleach, biocide and swimming pool treatment.
Air & Water Reactions
Decomposes in water forming chlorine and oxygen gases.
Reactivity Profile
Explodes when heated or by reaction with organic materials, including: carbon, carbon disulfide, ethers, hydrocarbons, dicyanogen, any readily oxidizable materials (ammonia, potassium, arsenic, antimony, sulfur, mercury sulfide, calcium phosphide, phosphine, phosphorus, hydrogen sulfide, antimony sulfide, barium sulfide, mercury sulfide, and tin sulfide). Dissolves in alkalis, forming a mixture of chlorite and chlorate. Concentration of gas should be limited to less than 10% to reduce explosion hazard. Alcohols are oxidized explosively.
Hazard
Although a nonflammable gas, it reacts explosively with many substances, including organics, metals, metal sulfides, sulfur, phosphorus, nitric oxide, ammonia, carbon disulfide, metal hydrides, and charcoal. It is a severe irritant to the eyes, nose, skin, and respiratory tract. Inhalation of the gas at 100 ppm can be fatal to humans.
Health Hazard
Chlorine monoxide is severely irritating tothe eyes, skin, and mucous membranes.Exposure can cause lung damage. LC50 dataare not available for this compound. Ashort exposure to 100 ppm concentration cancause death to humans.
Fire Hazard
Nonflammable gas. Chlorine monoxide is a highly reactive compound, exploding by itself when rapidly heated. Chlorine monoxide explodes with organic compounds, charcoal, metals, metal sulfides, sulfur, phosphorus, ammonia, nitric oxide, and carbon disulfide.
dichlorine oxide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 027-88013699 17354350817 | Ryan@jiutian-bio.com | China | 7433 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | 1046@dideu.com | China | 10008 | 58 |





