ChemicalBook >> CAS DataBase List >>BOC-12-ADO-OH
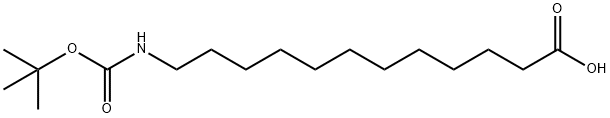
BOC-12-ADO-OH
- CAS No.
- 18934-81-1
- Chemical Name:
- BOC-12-ADO-OH
- Synonyms
- BOC-12-ADO-OH;BOC-NH-(CH2)11-COOH;BOC-12-AMINOLAURIC ACID;Boc-12-Ado-OH >=98.0% (TLC);BOC-12-AMINODODECANOIC ACID;12-(BOC-AMINO)DODECANOIC ACID;N-Boc-12-aminododecanoic acid;T-BUTOXYCARBONYL-12-AMINOLAURIC ACID;T-BUTOXYCARBONYL-12-AMINODODECANOIC ACID;N-tert-Butoxycarbonyl-12-aminododecanoic acid
- CBNumber:
- CB5104471
- Molecular Formula:
- C17H33NO4
- Molecular Weight:
- 315.45
- MDL Number:
- MFCD00235887
- MOL File:
- 18934-81-1.mol
- MSDS File:
- SDS
Last updated:2022-12-22 10:55:24
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P233-P260-P261-P264-P271-P280-P302+P352-P304-P304+P340-P305+P351+P338-P312-P321-P332+P313-P337+P313-P340-P362-P403-P403+P233-P405-P501 |
| WGK Germany | 3 |
BOC-12-ADO-OH price More Price(25)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 09780 | Boc-12-Ado-OH ≥98.0% (TLC) | 18934-81-1 | 500mg | $48.4 | 2024-03-01 | Buy |
| TRC | B602715 | Boc-12-aminododecanoicAcid | 18934-81-1 | 1g | $175 | 2021-12-16 | Buy |
| Usbiological | 235834 | Boc-12-aminododecanoic acid | 18934-81-1 | 1g | $200 | 2021-12-16 | Buy |
| Usbiological | 264982 | Boc-12-aminododecanoic acid | 18934-81-1 | 1g | $360 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | AAA0005019 | BOC-12-ADO-OH 95.00% | 18934-81-1 | 5G | $1018.71 | 2021-12-16 | Buy |
BOC-12-ADO-OH Chemical Properties,Uses,Production
Description
Boc-12-Ado-OH can be used as a PROTAC linker in the synthesis of PROTACs. Boc-12-Ado-OH is an alkane chain with terminal carboxlic acid and Boc-protected amino groups. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
BOC-12-ADO-OH Preparation Products And Raw materials
Raw materials
Preparation Products
BOC-12-ADO-OH Suppliers
Global( 59)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| BOC Sciences | +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19915 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| Sichuan Biosynce Pharmatech Co., Ltd. | +8619950309693 | diane@biosynce.com | China | 4874 | 58 |
| Jilin Chinese Academy of Sciences-yanshen Technology | +undefined18143011203 | info@chemextension.com | China | 42057 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| YangZhou Ruihong Biotech Co., Ltd. | 015380367604 15380367604 | China | 509 | 55 | |
| Shanghai SunSyn Pharmaceutical Co., Ltd. | -2408801818 18621020637 | sales@sunsynpharma.com | China | 293 | 55 |
| Bide Pharmatech Ltd. | 400-1647117 15221909166 | product02@bidepharm.com | China | 41438 | 60 |
View Lastest Price from BOC-12-ADO-OH manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-01-06 | BOC-12-ADO-OH
18934-81-1
|
US $0.80-1.80 / KG | 1KG | 95%~99.99% | 10 MT/ Month | Career Henan Chemica Co |
-

- BOC-12-ADO-OH
18934-81-1
- US $0.80-1.80 / KG
- 95%~99.99%
- Career Henan Chemica Co
18934-81-1(BOC-12-ADO-OH)Related Search:
12-(BOC-AMINO)DODECANOIC ACID
BOC-12-ADO-OH
BOC-12-AMINODODECANOIC ACID
BOC-12-AMINOLAURIC ACID
BOC-NH-(CH2)11-COOH
N-TERT-BUTYLOXYCARBONYL-12-AMINO-DODECANOIC ACID
T-BUTOXYCARBONYL-12-AMINODODECANOIC ACID
T-BUTOXYCARBONYL-12-AMINOLAURIC ACID
N-T-BUTYLOXYCARBONYL-12-AMINO-DODECANOIC ACID
N-tert-Butoxycarbonyl-12-aminododecanoic acid
Boc-12-Ado-OH >=98.0% (TLC)
N-Boc-12-aminododecanoic acid
12-{[(tert-butoxy)carbonyl]amino}dodecanoic acid
Dodecanoic acid, 12-[[(1,1-dimethylethoxy)carbonyl]amino]-
12-[(2-methylpropan-2-yl)oxycarbonylamino]dodecanoic acid
18934-81-1
C17H33NO4
Specialty Synthesis
Unnatural Amino Acid Derivatives
Peptide Synthesis
Linear Core Amino Acids
Amino Acids