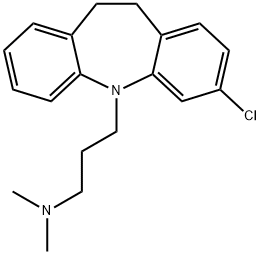
Clomipramine
- CAS No.
- 303-49-1
- Chemical Name:
- Clomipramine
- Synonyms
- CLOMIPRAMINE HCL;ANAFRANIL;CHLORIMIPRAMINE;CIM;g34586;G34596;5H-Dibe;G 34586;Hydiphen;CLOMIPRAMINE
- CBNumber:
- CB5173107
- Molecular Formula:
- C19H23ClN2
- Molecular Weight:
- 314.85
- MDL Number:
- MFCD00242755
- MOL File:
- 303-49-1.mol
- MSDS File:
- SDS
| Melting point | 189.5°C |
|---|---|
| Boiling point | bp0.3 160-170° |
| Density | 1.0568 (rough estimate) |
| refractive index | 1.5749 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | H2O: 25 mg/mL |
| form | powder |
| pka | pKa 9.38(H2O) (Uncertain) |
| color | white to off-white |
| CAS DataBase Reference | 303-49-1(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | NUV44L116D |
| ATC code | N06AA04 |
| NIST Chemistry Reference | Clomipramine(303-49-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H410-H302-H370-H336 |
| Precautionary statements | P260-P264-P270-P307+P311-P321-P405-P501-P264-P270-P301+P312-P330-P501-P261-P271-P304+P340-P312-P403+P233-P405-P501-P273-P391-P501 |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22 |
| Safety Statements | 36 |
| WGK Germany | 3 |
| RTECS | HN9055000 |
Clomipramine price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| AK Scientific | R900 | Clomipramine | 303-49-1 | 100mg | $204 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 16831 | Clomipramine | 303-49-1 | 1mg | $520 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0002067 | CLOMIPRAMINE 95.00% | 303-49-1 | 1G | $975.45 | 2021-12-16 | Buy |
| Crysdot | CD11149548 | 3-(3-Chloro-10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N,N-dimethylpropan-1-amine 97% | 303-49-1 | 1g | $455 | 2021-12-16 | Buy |
| Alichem | 303491 | 3-(3-Chloro-10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N,N-dimethylpropan-1-amine | 303-49-1 | 1g | $460 | 2021-12-16 | Buy |
Clomipramine Chemical Properties,Uses,Production
Originator
Anafranil,Ciba Geigy,Switz.,1968
Uses
Antidepressan.
Definition
ChEBI: A dibenzoazepine that is 10,11-dihydro-5H-dibenzo[b,f]azepine which is substituted by chlorine at position 3 and in which the hydrogen attached to the nitrogen is replaced by a 3-(dimethylamino)propyl group. One of the more sedating tricyclic antidepressan s, it is used as the hydrochloride salt for the treatment of depression as well as obsessive-compulsive disorder and phobias.
Manufacturing Process
22.9 parts of 3-chloroiminodibenzyl are dissolved in 300 parts by volume of xylene, and 4 parts of sodium amide, pulverized and suspended in toluene,are added thereto while stirring and maintaining the whole under a nitrogen atmosphere. The xylene solution immediately turns dark colored, but upon crystallization of the sodium salt therefrom it becomes again light-colored. The reaction mixture is stirred for about 2 hours at 80°C until the development of ammonia has terminated. A solution of γ-dimethylaminopropyl chloride in toluene, prepared by setting free a corresponding amount of the free base from 17.4 parts of its hydrochloride salt by addition of aqueous sodium hydroxide solution in about 10% excess, extraction with toluene and drying for 2 hours over anhydrous sodium sulfate is added to the xylene solution containing the sodium salt mentioned above and the whole is stirred under reflux for 15 hours. Precipitated sodium chloride is filtered off and the filtrate is concentrated. The residue is diluted with ether, and the hydrochloride of 3- chloro-5-(γ-dimethylaminopropyl)-iminodibenzyl is precipitated by introducing dry, gaseous hydrogen chloride. It is filtered off under suction and purified by repeated recrystallization from acetone; the pure substance melts at 191.5°C to 192°C.
brand name
Anafranil (Tyco).
Therapeutic Function
Antidepressant
Mechanism of action
Clomipramine is different from the other TCAs, exhibiting preferential selectivity for inhibiting the reuptake of
5-HT at the presynaptic neuronal membrane. Its antidepressant mechanism of action as an inhibitor of the
5-HT transporter is reduced in vivo, however, because of the formation of its active metabolite,
N-desmethylclomipramine, which inhibits the reuptake of NE. As a result of its common structure with the
other TCAs, clomipramine shares the pharmacological and adverse-effect profile of the other TCAs.
The efficacy of clomipramine relative to the other TCAs in the treatment of obsessive-compulsive disorder
may be related to its potency in blocking 5-HT reuptake at the presynaptic neuronal membrane, suggesting a
dysregulation of 5-HT for the pathogenesis of obsessive-compulsive disorder. Clomipramine appears to
decrease the turnover of 5-HT in the CNS, probably because of a decrease in the release and/or synthesis of
5-HT.
Although in vitro studies suggest that clomipramine is approximately four times more potent than fluoxetine as
a 5-HT reuptake inhibitor, in vivo studies suggest the opposite. This difference has been attributed to the
relatively long elimination half-lives for fluoxetine and its principal serotonergic metabolite norfluoxetine. In
addition, metabolism of clomipramine to its N-desmethyl secondary amine metabolite decreases the potency
and selectivity of 5-HT -reuptake inhibition of clomipramine, but not fluoxetine.
Pharmacokinetics
Clomipramine appears to be well absorbed from the GItract following oral administration, with an oral
bioavailability of approximately 50%, suggesting some first-pass metabolism. Food does not
appear to substantially affect its bioavailability. Clomipramine and its active metabolite,
N-desmethylclomipramine, exhibit nonlinear pharmacokinetics at 25 to 150 mg daily. At dosages exceeding
150 mg daily, their elimination half-lives may be considerably prolonged, allowing plasma concentrations of
both to accumulate, which may increase the incidence of plasma concentration-dependent adverse effects,
particularly seizures. Because of the relatively long elimination half-lives of clomipramine and
N-desmethylclomipramine, their steady-state plasma concentrations generally are achieved within
approximately 1 to 2 weeks. Plasma concentrations of N-desmethylclomipramine generally are greater than
those for chlomipramine at steady-state conditions. Clomipramine crosses the placenta and is distributed into
breast milk.
Clomipramine is primarily metabolized by CYP2D6 N-dealkylation to its pharmacologically active metabolite,
the 2- and 8-hydroxylated metabolites and their glucuronides, and clomipramine N-oxide.
N-dealkylation also involves CYP3A4, CYP2C19, CYP2C9, and CYP1A2. Like all the other secondary amine
TCAs, N-desmethylclomipramine is significantly more potent as an inhibitor of NE reuptake than clomipramine
is. Although N-desmethylclomipramine is pharmacologically active, its efficacy in obsessive-compulsive
disorder is not known. 8-Hydroxyclomipramine and 8-hydroxydesmethylclomipramine also are
pharmacologically active, but their clinical importance remains unknown. The hydroxylation and
N-demethylation of clomipramine highlight CYP2D6 polymorphism in healthy adults who were phenotyped as either extensive metabolizers or poor metabolizers of clomipramine. Interindividual variation in plasma
concentrations may be caused by genetic differences in the metabolism of the drug. In addition, CYP1A2 ring
hydroxylates clomipramine. Less than 1% of an oral dose of clomipramine was excreted unmetabolized into
the urine, with 8-hydroxyclomipramine glucuronide as the principal metabolite found in the urine. The effects
of renal clearance suggest that clomipramine and desmethylclomipramine should be decreased in patients
with renal impairment.
Clinical Use
Clomipramine is considered to be the most powerful antidepressant ever made. This dihydrodibenzazepine TCA, with actions on both the NE and 5-HT transporters, was the last of the major TCAs to come to market. Initially, the U.S. FDA regarded it as another “me-too” drug, and accordingly, they did not license it. Subsequently, however, it was licensed for the treatment of obsessive-compulsive disorders. Clomipramine differs from imipramine only by the addition of a 3-chloro group.
Side effects
Male patients taking clomipramine should be informed of sexual dysfunction as a side effect associated with antidepressants having significant serotonergic activity. Sexual dysfunction in men appears as ejaculatory incompetence, ejaculatory retardation, decreased libido, or inability to obtain or maintain an erection. Sexual dysfunction is dose-related and may be treated by simply lowering the drug dose.
Clomipramine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | contact@fuxinpharm.com | China | 10297 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
Related articles
- Clomipramine: Uses, Treatments, Drug interactions and Side effects
- Clomipramine is a safe, reliable and rapid-acting tricyclic antidepressant which is white or yellowish crystalline powder at r....
- Oct 22,2019
View Lastest Price from Clomipramine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-27 | Clomipramine USP/EP/BP
303-49-1
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2021-07-02 | Clomipramine
303-49-1
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2019-07-06 | Clomipramine
303-49-1
|
US $1.00 / KG | 1KG | 98% | 1kg,5kg,100kg | Career Henan Chemical Co |
-

- Clomipramine USP/EP/BP
303-49-1
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- Clomipramine
303-49-1
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Clomipramine
303-49-1
- US $1.00 / KG
- 98%
- Career Henan Chemical Co