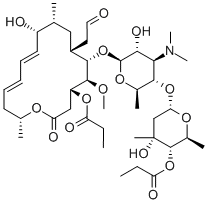
MIDECAMYCIN
- CAS No.
- 35457-80-8
- Chemical Name:
- MIDECAMYCIN
- Synonyms
- medemycin;mydecamycin;medecamycin;sf837;Aboren;Myoxam;Midecin;Momicin;Macropen;MoMicine
- CBNumber:
- CB5175530
- Molecular Formula:
- C41H67NO15
- Molecular Weight:
- 813.97
- MDL Number:
- MFCD00865000
- MOL File:
- 35457-80-8.mol
- MSDS File:
- SDS
| Melting point | 155℃ -156℃ |
|---|---|
| Boiling point | 874℃ |
| alpha | D23 -67° (c = 1 in ethanol) |
| Density | 1.1651 (rough estimate) |
| refractive index | 1.6220 (estimate) |
| Flash point | >110°(230°F) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 6.9 in 50% aq ethanol |
| color | White to Off-White |
| Water Solubility | Soluble in methanol or ethanol. Insoluble in water |
| FDA UNII | N34Z0Y5UH7 |
| ATC code | J01FA03 |
SAFETY
Risk and Safety Statements
| Hazard statements | H413 |
|---|---|
| RTECS | OH4730600 |
MIDECAMYCIN price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Alfa Aesar | J66046 | Midecamycin, 900 μg/mg | 35457-80-8 | 500mg | $180 | 2024-03-01 | Buy |
| Alfa Aesar | J66046 | Midecamycin, 900 μg/mg | 35457-80-8 | 1g | $258 | 2024-03-01 | Buy |
| TRC | M343250 | Midecamycin(>85%) | 35457-80-8 | 10g | $1190 | 2021-12-16 | Buy |
| Usbiological | 017648 | Midecamycin | 35457-80-8 | 1g | $460 | 2021-12-16 | Buy |
| Usbiological | 257342 | Midecamycin | 35457-80-8 | 500mg | $468 | 2021-12-16 | Buy |
MIDECAMYCIN Chemical Properties,Uses,Production
Description
Midecamycin, a macrolide antibiotic having a 16-membered lactone ring, was found in the culture broth of Streptomyces mycarofaciens by Meiji Seika Kaisha in 1971. Under specific culture conditions, it is produced by the organism as a single component. Midecamycin shows almost the same antimicrobial spectrum and activity as kitasamycin. Although its serum and urine concentrations are low, it distributes in tissues at high concentration following oral administration.
Chemical Properties
White Solid
Originator
Medemycin,Meiji Seika,Japan,1974
Uses
A broad spectrum antibiotic
Uses
A 16-membered ring macrolide antibiotic used in ophthalmology as well as other medical fields.
Definition
ChEBI: Midecamycin is an organic molecular entity.
Manufacturing Process
The SF-837 strain, namely Streptomyces mycarofaciens identified as ATCC No.
21454 was inoculated to 60 liters of a liquid culture medium containing 2.5%
saccharified starch, 4% soluble vegetable protein, 0.3% potassium chloride
and 0.3% calcium carbonate at pH 7.0, and then stir-cultured in a jarfermenter at 28°C for 35 hours under aeration. The resulting culture was
filtered directly and the filter cake comprising the mycelium cake was washed
with dilute hydrochloric acid.
The culture filtrate combined with the washing liquid was obtained at a total
volume of 50 liters (potency 150 mcg/ml). The filtrate (pH 8) was then
extracted with 25 liters of ethyl acetate and 22 liters of the ethyl acetate
phase was concentrated to approximately 3 liters under reduced pressure. The
concentrate was diluted with 1.5 liters of water, adjusted to pH 2.0 by addition
of 5N hydrochloric acid and then shaken thoroughly. The aqueous phase was
separated from the organic phase and this aqueous solution was adjusted to
pH 8 by addition of 3N sodium hydroxide and then extracted with 800 rnl of
ethyl acetate. The resulting ethyl acetate extract was then shaken similarly
together with 500 ml of aqueous hydrochloric acid to transfer the active
substances into the latter which was again extracted with 400 ml of ethyl
ether at pH 8.The ether extract was dried with anhydrous sodium sulfate and
concentrated under reduced pressure to give 16.5 g of light yellow colored
powder.
12 g of this crude powder were dissolved in 200 ml of ethyl acetate and the
solution was passed through a column of 600 ml of pulverized carbon which
had been impregnated with ethyl acetate. The development was carried out
using ethyl acetate as the solvent and the active fractions of eluate were
collected to a total volume of 2,500 ml, which was then evaporated to dryness
under reduced pressure to yield 5 g of a white colored powder. This powder
was dissolved in 10 ml of benzene and the insoluble matters were filtered out.
The filtered solution in benzene was then subjected to chromatographic
isolation by passing through a column of 700 ml of silica gel which had been
impregnated with benzene. The development of the active substances
adsorbed on the silica gel was effected using a solvent system consisting of
benzene-acetone (4:1), and the eluate was collected in fractions of each 20
ml. The active fractions No. 90-380 which gave a single spot in alumina thin
layer chromatography and which could be recognized as containing the SF-
837 substance purely in view of the Rf-value of the single spot were combined
together to a total volume of 4,000 ml, and then concentrated under reduced
pressure to yield 1.5 g of white colored powder of a melting point of 122°C to
124°C which was found by analysis to be the pure SF-837 substance free
base.
Therapeutic Function
Antibacterial
Pharmaceutical Applications
A naturally occurring metabolite of Streptomyces mycarofaciens, supplied as the native compound and as midecamycin acetate for oral administration.
It is rapidly and extensively metabolized and is said to exhibit less toxicity than earlier macrolides. It is of limited availability.
MIDECAMYCIN Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| .GZ HONESTCHEM CO.,LTD | +86-15013270415 | honestchemical@foxmail.com | China | 247 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3791 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9553 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29474 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9427 | 58 |
View Lastest Price from MIDECAMYCIN manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
|
|
2021-11-02 | MIDECAMYCIN
35457-80-8
|
US $238.10 / g | 100g | 99% | 200kg | Baoji Guokang Healthchem co.,ltd | |
 |
2021-07-20 | Midecamycin
35457-80-8
|
US $1.00-1.00 / KG | 1g | 99% | 50tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2021-07-09 | Midecamycin
35457-80-8
|
US $1.00 / g | 300g | 99.8% | 20 TONS | .GZ HONESTCHEM CO.,LTD |
-
- MIDECAMYCIN
35457-80-8
- US $238.10 / g
- 99%
- Baoji Guokang Healthchem co.,ltd
-

- Midecamycin
35457-80-8
- US $1.00-1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
-

- Midecamycin
35457-80-8
- US $1.00 / g
- 99.8%
- .GZ HONESTCHEM CO.,LTD