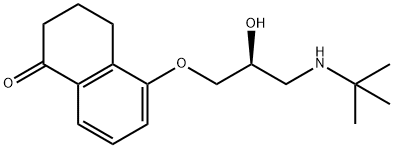
LEVOBUNOLOL
- CAS No.
- 47141-42-4
- Chemical Name:
- LEVOBUNOLOL
- Synonyms
- W 6421A;AK-Beta;Betegan;l-Bunolol;Liquifilm;LEVOBUNOLOL;Levobunololum;LEVOBUNOLOL USP/EP/BP;[(-)-5-[3,(t-Butylamino)-2-hydroxy-propoxy]-3,4-di,HCl;5-[3-(tert-Butylamino)-2β-hydroxypropyloxy]-3,4-dihydronaphthalen-1(2H)-one
- CBNumber:
- CB5210167
- Molecular Formula:
- C17H25NO3
- Molecular Weight:
- 291.39
- MDL Number:
- MFCD00864586
- MOL File:
- 47141-42-4.mol
- MSDS File:
- SDS
| Melting point | 209-211°C |
|---|---|
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| CAS DataBase Reference | 47141-42-4(CAS DataBase Reference) |
| FDA UNII | G6317AOI7K |
| ATC code | S01ED03 |
SAFETY
Risk and Safety Statements
| Toxicity | LD50 in male, female rats, mice (mg/kg): 700, 800, 1530, 1220 orally; 25, 28, 78, 84 i.v.; LD50 in male, female hamsters, dogs (mg/kg): 435, 500, 100, 100 orally (Kaplan, 1980) |
|---|
LEVOBUNOLOL price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | API0003156 | LEVOBUNOLOL 95.00% | 47141-42-4 | 1G | $630 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 11354 | LevobunololHCl | 47141-42-4 | 1.25g | $1560 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0003156 | LEVOBUNOLOL 95.00% | 47141-42-4 | 2.5G | $1947 | 2021-12-16 | Buy |
| AHH | MT-53190 | Levobunolol 98% | 47141-42-4 | 5g | $510 | 2021-12-16 | Buy |
| Chemenu | CM129099 | (S)-5-(3-(tert-butylamino)-2-hydroxypropoxy)-3,4-dihydronaphthalen-1(2H)-one 95% | 47141-42-4 | 1g | $327 | 2021-12-16 | Buy |
LEVOBUNOLOL Chemical Properties,Uses,Production
Chemical Properties
White Solid
Originator
Betagan,Allergan,India
Uses
Levobunolol hydrochloride is an antiglaucoma agent in ophthalmie solutions.
Uses
As a non-selective adrenoceptor antagonist, Levobunolol is used as an anticonvulsant.
Definition
ChEBI: A cyclic ketone that is 3,4-dihydronaphthalen-1-one substituted at position 5 by a 3-(tert-butylamino)-2-hydroxypropoxy group (the S-enantiomer). A non-selective beta-adrenergic antagonist used (as its hydrochlo ide salt) for treatment of glaucoma.
Manufacturing Process
9.62 g (59 mmoles) 5-hydroxy-3,4-dihydro-1(2H)-naphthalenone, 67 ml
toluene, 0.36 g (1.1 mmoles) tetra-n-butylammonium bromide, 4.51 g (68
mmoles) 85% potassium hydroxide and 20 ml (254 mmoles) (R)-(-)-
epichlorhydrine were placed in an appropriate flask fitted with efficient
mechanical stirring, and the mixture was heated under reflux for two hours.
The mixture was allowed to cool to 30°C, 50 ml toluene and 50 ml water were
added and the mixture was vigorously stirred. The organic phase was
removed and the aqueous phase extracted with 25 ml toluene. The combined
organic phases were concentrated at reduced pressure, 31 ml (300 mmoles)
tert-butylamine, 45 ml ethanol and 3 ml deionized water were added, and the
solution was heated under reflux for one hour. The mixture was allowed to
cool to 40°C and the volatile products were distilled at reduced pressure.
Toluene (9 ml) was added to the residue and volatiles were distilled at
reduced pressure. (S)-5-(2,3-Epoxypropoxy)-3,4-dihydro-1(2H)-
naphthalenone with an optical purity greater than 95% was obtained. Toluene
(75 ml) was added to the product, and then, 10 ml of 35% (w/v) hydrochloric
acid and 110 ml water, and the mixture was stirred for fifteen minutes. The
organic phase was decanted and the aqueous one was extracted with 50 ml
toluene. The aqueous phase was basified by addition of a solution of 5.1 g
sodium hydroxide in 150 ml water and extracted twice with toluene (100 and
50 ml, respectively). The combined organic extracts were dried with
anhydrous sodium sulfate, decolorized with active charcoal and filtered.
To the above toluenic solution containing levobunolol as free base, 16 ml
ethanol and the stoichiometric amount of hydrogen chloride were added. The
stirred mixture was cooled below 10°C and kept at this temperature for one
hour. The precipitated solid was filtered, washed with toluene, recrystallized
twice from 43 ml ethanol and dried to give 10.0 g (51% yield) of (-)-3,4-
dihydro-5-(3-(tert-butylamino)-2-hydroxypropoxy)-1(2H)-naphthalenone
hydrochloride (levobunolol hydrochloride) having a rotary power at 25°C
below -19°.
brand name
Akbeta(Akorn); Betagan(Allergan).
Therapeutic Function
Beta-adrenergic blocker
LEVOBUNOLOL Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3683 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9409 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29322 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| LGM Pharma | 1-(800)-881-8210 | inquiries@lgmpharma.com | United States | 2127 | 70 |
| Chemsky (shanghai) International Co.,Ltd | 021-50135380 | shchemsky@sina.com | China | 15421 | 60 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12338 | 58 |
| Shanghai Han-Xiang Chemical Co., Ltd. | 15971444841 | amber@biochempartner.com | China | 3063 | 58 |
View Lastest Price from LEVOBUNOLOL manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-08-12 | LEVOBUNOLOL USP/EP/BP
47141-42-4
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2021-07-20 | Levobunolol
47141-42-4
|
US $1.00-1.00 / KG | 1g | 99% | 50tons | Shaanxi Dideu Medichem Co. Ltd |
-

- LEVOBUNOLOL USP/EP/BP
47141-42-4
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- Levobunolol
47141-42-4
- US $1.00-1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
47141-42-4(LEVOBUNOLOL)Related Search:
1of4