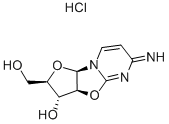
2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
- CAS No.
- 10212-25-6
- Chemical Name:
- 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
- Synonyms
- Cyclo-C;nsc-145668;Cyclo-Cr·HCl;Anhydro-.HCl;Anhydro-Cr.HCl;NSC 145668 HCl;ANCITABINE HCL;CYCLOCYTIDINE HCL;Cyclocytidine HC1;2,2'-Anhydro-C.Hcl
- CBNumber:
- CB5292165
- Molecular Formula:
- C9H12ClN3O4
- Molecular Weight:
- 261.66
- MDL Number:
- MFCD00012636
- MOL File:
- 10212-25-6.mol
| Melting point | 269-270 °C (dec.)(lit.) |
|---|---|
| alpha | -21.8 º (c=2,water) |
| refractive index | -21 ° (C=2, H2O) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Methanol (Slightly), Water (Sparingly) |
| form | Powder |
| color | White |
| Merck | 14,629 |
| InChIKey | KZOWNALBTMILAP-JBMRGDGGSA-N |
| LogP | -2.301 (est) |
| FDA UNII | 3T6920M469 |
| NCI Drug Dictionary | ancitabine hydrochloride |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Safety Statements | 24/25 |
| WGK Germany | 2 |
| RTECS | LV2615000 |
| HS Code | 29213000 |
2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A8598 | Ancitabine hydrochloride | 10212-25-6 | 1g | $108 | 2024-03-01 | Buy |
| TCI Chemical | C2207 | 2,2'-O-Cyclocytidine Hydrochloride >98.0%(HPLC)(N) | 10212-25-6 | 1g | $48 | 2024-03-01 | Buy |
| TCI Chemical | C2207 | 2,2'-O-Cyclocytidine Hydrochloride >98.0%(HPLC)(N) | 10212-25-6 | 5g | $139 | 2024-03-01 | Buy |
| Alfa Aesar | J63845 | Cyclocytidine hydrochloride, 98+% | 10212-25-6 | 1g | $51.65 | 2024-03-01 | Buy |
| Alfa Aesar | J63845 | Cyclocytidine hydrochloride, 98+% | 10212-25-6 | 5g | $180 | 2021-12-16 | Buy |
2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride Chemical Properties,Uses,Production
Chemical Properties
white fine crystalline powder
Originator
Cyclo-C,Kohjin,Japan,1975
Uses
Ancitabine Hydrochloride is an antineoplastic agent.
Uses
Anti-tumor agent
Definition
ChEBI: A hydrochloride salt resulting from the reaction of equimolar amounts of ancitabine and hydrogen chloride.
Manufacturing Process
A series of reaction steps may be employed in which: (1) Uridine is reacted
with trityl chloride to give 5'-o-trityluridine; (2) Imidazole is reacted with
thiophosgene and that product reacted with the 5'-o-trityluridine to give 2,2'-
anhydro-1-(5'-o-trityl-β-D-arabinofuranosyl)uracil; (3) The preceding uracil
product is converted to the thiouracil using hydrogen sulfide; (4) The trityl
group is removed by treatment with 80% acetic acid; (5) A triacetylated
product is obtained using acetic anhydride; (6) A dithiouracil is prepared from
the uracil intermediate using phosphate pentasulfide.
Preparation of 1-(β-D-arabinofuranosyl)-2-thiocytosine: A solution of 2.0 g of
1-(2',3',5'-O-triacetyl-β-D-arabinofuranosyl)-2,4-dithiouracilin 100 ml of
methanol is saturated with anhydrous ammonia at 0°C. The mixture, in a
glass liner, is heated in a pressure bomb at 100°C for three hours. The
reaction mixture is concentrated to a gum in vacuum, and most of the
byproduct acetamide is removed by sublimation at 60°C/0.1 mm. The residue
is chromatographed on 100 g of silica gel. Elution of the column with
methylene chloride-methanol mixtures with methanol concentrations of 2-25%
gives fractions containing acetamide and a series of brown gums. The desired
product is eluted with 30% methanol-methylene chloride to give a total yield
of 0.386 g (30%), MP 175-180°C (dec.). Recrystallization from methanolisopropanol
furnishes an analytical sample, MP 180-182°C (dec.).
To a solution of 80 mg of 1-(β-D-arabinofuranosyl)-2-thiocytosine in 12 ml of
water is added dropwise 3 ml of a 1 M bromine solution in carbon
tetrachloride. At this point the color of the bromine persists for about 2-3
minutes after each addition. The unreacted bromine is blown off with a stream
of nitrogen, and the reaction mixture is concentrated to a syrup in vacuum
using a bath temperature less than 50°C. The residue is evaporated three
times with 10 ml portions of ethanol, whereupon it crystallizes. The product is
triturated with cold ethanol and with ether to obtain 17 mg of 2,2'-anhydro-1-
(β-D-arabinofuranosyl)cytosine hydrobromide, MP 240°C (dec.).
Treatment of the hydrobromide with a slight excess of ethanolic ammonia
yields the base which may then be converted to the hydrochloride.
Therapeutic Function
Antineoplastic
2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 951 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 1696 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | figo.gao@foxmail.com | China | 6992 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
View Lastest Price from 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
10212-25-6
|
US $50.00 / kg | 1kg | 99.10% | 50000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-22 | 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
10212-25-6
|
US $100.00 / kg | 1kg | 99% | 2000kg | hebei hongtan Biotechnology Co., Ltd | |
 |
2024-04-22 | 2,2'-Anhydro- 1-beta-D- arabinofuranosylcytosine hydrochloride
10212-25-6
|
US $100.00 / kg | 1kg | 99.9% | 1000 | hebei hongtan Biotechnology Co., Ltd |
-

- 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
10212-25-6
- US $50.00 / kg
- 99.10%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
10212-25-6
- US $100.00 / kg
- 99%
- hebei hongtan Biotechnology Co., Ltd
-

- 2,2'-Anhydro- 1-beta-D- arabinofuranosylcytosine hydrochloride
10212-25-6
- US $100.00 / kg
- 99.9%
- hebei hongtan Biotechnology Co., Ltd
2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride Spectrum
10212-25-6(2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride)Related Search:
1of4