Upadacitinib
- CAS No.
- 1310726-60-3
- Chemical Name:
- Upadacitinib
- Synonyms
- ABT-494;(3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide;upatinib;Upadacitinib (ABT-494);benzyl (4S)-3-(N-(ethoxycarbonyl)-N-(5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-yl)glycyl)-4-ethylpyrrolidine-1-carboxylate;1-Pyrrolidinecarboxamide, 3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)-, (3S,4R)-;(3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide, Upadacitinib;CS-2730;Upadacitinb;Upadacitinib
- CBNumber:
- CB53121345
- Molecular Formula:
- C17H19F3N6O
- Molecular Weight:
- 380.37
- MDL Number:
- MFCD30502663
- MOL File:
- 1310726-60-3.mol
- MSDS File:
- SDS
| Density | 1.56±0.1 g/cm3(Predicted) |
|---|---|
| storage temp. | Store at -20°C |
| solubility |
DMSO:42.67(Max Conc. mg/mL);112.17(Max Conc. mM) DMSO:PBS (pH 7.2) (1:1):0.5(Max Conc. mg/mL);1.31(Max Conc. mM) DMF:30.0(Max Conc. mg/mL);78.87(Max Conc. mM) Ethanol:76.0(Max Conc. mg/mL);199.8(Max Conc. mM) |
| form | A crystalline solid |
| pka | 11.89±0.60(Predicted) |
| InChI | InChI=1S/C17H19F3N6O/c1-2-10-7-25(16(27)24-9-17(18,19)20)8-11(10)13-5-22-14-6-23-15-12(26(13)14)3-4-21-15/h3-6,10-11,21H,2,7-9H2,1H3,(H,24,27)/t10-,11+/m1/s1 |
| InChIKey | WYQFJHHDOKWSHR-MNOVXSKESA-N |
| SMILES | N1(C(NCC(F)(F)F)=O)C[C@H](C2N3C4C=CNC=4N=CC3=NC=2)[C@H](CC)C1 |
| FDA UNII | 4RA0KN46E0 |
| ATC code | L04AA44 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
Upadacitinib price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 29706 | Upadacitinib | 1310726-60-3 | 5mg | $116 | 2024-03-01 | Buy |
| Cayman Chemical | 29706 | Upadacitinib | 1310726-60-3 | 25mg | $381 | 2024-03-01 | Buy |
| Cayman Chemical | 29706 | Upadacitinib | 1310726-60-3 | 1mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 29706 | Upadacitinib | 1310726-60-3 | 10mg | $169 | 2024-03-01 | Buy |
| TRC | U800075 | Upadacitinib | 1310726-60-3 | 1mg | $300 | 2021-12-16 | Buy |
Upadacitinib Chemical Properties,Uses,Production
Introduction
Upadacitinib (ABT-494) is a Janus kinase 1 (JAK1) inhibitor currently being developed by AbbVie for the treatment of rheumatoid arthritis (RA), Crohn’s disease, ulcerative colitis, atopic dermatitis, and psoriatic arthritis. It is also being investigated as a potential treatment for people with active ankylosing spondylitis (AS).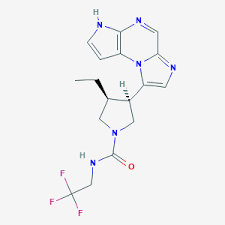
AS is an inflammatory, progressive autoimmune disease that affects the joints of the spine. As the disease progresses, calcium deposits form where ligaments attach to the bones that form the spine, leading to reduced flexibility of the back.
Description
Upadacitinib is a JAK1 inhibitor (IC50 = 47 nM). It is selective for JAK1 over JAK3 and tyrosine kinase 2 (Tyk2; IC50s = 2,304 and 4,690 nM, respectively), as well as a panel of 83 additional kinases at 1 μM, but does inhibit JAK2, Rho-associated kinase I (ROCK1), and ROCK2 (IC50s = 120, 920, and 430 nM, respectively). Upadacitinib decreases cytokine-induced STAT phosphorylation in a variety of human cells with IC50 values ranging from 1.6 to 649 nM. It reduces M. tuberculosis-induced paw swelling and bone erosion in a rat model of arthritis when administered at doses of 1, 3, and 10 mg/kg twice per day for 17 days. Formulations containing upadacitinib have been used in the treatment of rheumatoid arthritis.
Uses
Upadacitinib also known as ABT-494, is a potent and selective Janus kinase (JAK) 1 inhibitor being developed for the treatment of several autoimmune disorders, Janus kinase inhibitors for rheumatoid arthritis. It is used to treat moderate to severely active rheumatoid arthritis, active psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis (a type of arthritis in the spine) with objective signs of swelling, moderate to severe ulcerative colitis, and moderate to severe Crohn's disease in patients who have taken other medicines (eg, methotrexate) that did not work well. It is also used to treat moderate to severe atopic dermatitis (eczema) in patients who have taken other medicines that did not work well and whose condition is not well controlled with other treatments or in patients who cannot tolerate these treatments. It is also used to treatpsoriatic arthritis, axial SpA and Giant Cell Arteritis and Takayasu Arteritis.
brand name
Upadacitinib is marketed under the brand name RINVOQ for oral administration.
Biological Activity
Upadacitinib is a potent, orally active and selective Janus kinase 1 (JAK1) inhibitor (IC50=43 nM). Upadacitinib displays approximately 74 fold selective for JAK1 over JAK2 (200 nM) in cellular assays dependent on specific, relevant cytokines. Upadacitinib can be used for several autoimmune disorders research. In vivo, Upadacitinib inhibited paw swelling and bone destruction in a rat model of arthritis.
Mechanism of action
The Janus kinases (JAKs) are a family of cytoplasmic tyrosine kinases whose function is to transduce cytokine-mediated signals via the JAK-STAT pathway. There are four JAK subtypes, each of which has overlapping receptor responsibilities. Inhibitors of this enzyme family (jakinibs) have shown efficacy in treating certain inflammatory and autoimmune diseases such as rheumatoid arthritis and Crohn's disease. However, the first generation of these drugs, tofacitinib and ruxolitinib, lacked subtype selectivity, affecting JAK1/JAK3 and JAK1/JAK2 respectively. This has led to dose-limiting side effects in this otherwise promising class of drugs. Upadacitinib is a second generation Janus kinase inhibitor that is selective for the JAK1 subtype of this enzyme over the JAK2 (74-fold), JAK3 (58-fold) and tyrosine kinase 2 subtypes.
Clinical Use
Upadacitinib is indicated for the treatment of moderate to severe active rheumatoid arthritis in adults who have responded inadequately to, or who are intolerant to one or more disease-modifying antirheumatic drugs (DMARDs). Upadacitinib may be used as monotherapy or in combination with methotrexate.
Side effects
Common side effects are upper respiratory tract infections such as common cold and sinus infections (13.5% of patients in studies), nausea (3.5%), cough (2.2%), fever, and increased liver enzymes. Serious side effects include infections, including life-threatening ones, such as pneumonia, cellulitis, tuberculosis, as well as shingles and other herpes infections.
Synthesis
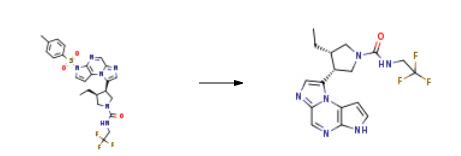
To a solution of (3S,4R)-3-ethyl-4-(3-tosyl-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazin-8-yl)- N-(2,2,2-trifluoroethyl)pyrrolidine-l-carboxamide (10 g) in tetrahydrofurane (50 ml_), a 10% solution of sodium hydroxide in water (15 ml) was added and heated to 50??C. The reaction mixture was stirred for 5 hours and cooled to 25??C. A saturated solution of sodium chloride (100 ml_) was added to the reaction mixture and extracted with dichloromethane (100 ml). The organic phase was separated and washed with water (100 ml_). An 2.5 % aqueous solution of HCI (100 ml) was added and stirred for 30 minutes. The organic phase was removed and the resultant acid aqueous phase was extracted with dichloromethane (100 ml). The final aqueous phase was cooled down to 0/5 ??C and a 10% solution of NaOH was charged until pH 10/12. The resultant suspension was filtered and washed with water (2 x 50 ml_). The cake was drained and dried at 30/40??C under vacuum to give an almost white amorphous solid. Yield: 80%. 1 H NMR (400MHz, d-DMSO) 612.27 (s,1H), 8.58 (s,1H), 7.47-7.43 (m,2H), 7.00-6.94 (m,2H), 4.38 -4.33 (m,1H), 3.92-3.67 (m,5H), 3.33-3.25 (m,1 H), 2.59-2.54 (m,1 H), 1.14-1.08 (m,1 H), 0.86-0.78 (m, 1H), 0.65-0.62 (m, 3H).
storage
Store at -20°C
Upadacitinib Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Rochi Pharmaceutical Co.,Ltd. | 21-38751876 +8615000076078 | info@rochipharma.com | China | 431 | 58 |
| Beijing Mesochem Technology Co.,Ltd | +8613651027935 | rachel@mesochem.com | China | 191 | 58 |
| TAIZHOU YUXIN BIOTECHNOLOGY CO,.LTD | +86-576-88902229;+86-0576-88902229 +8613968687450 | yuxin@yuxchem.com | China | 122 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| SHANDONG BOYUAN PHARMACEUTICAL CO., LTD. | +86-0531-69954981 +8615666777973 | dwyane.wang@boyuanpharm.com | China | 211 | 58 |
| Hebei Weimiao Import and Export Trade Co., Ltd. | +undefined19948166995 | sale01@hbweimiao.com | China | 67 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
Related articles
- Upadacitinib: Uses; Mechanism of Action; Administration and Contraindications
- Upadacitinib is FDA-approved for the treatment of moderate to severe rheumatoid arthritis (RA) that has not responded to first....
- Dec 19,2023
- Synthesis and Clinical study of Upadacitinib
- Upadacitinib, a JAK1 inhibitor, has shown good efficacy in clinical trials for the treatment of a variety of autoimmune and in....
- Oct 18,2022
View Lastest Price from Upadacitinib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Upadacitinib
1310726-60-3
|
US $300.00 / kg | 1kg | 98% | 200kg per month | Anhui Dexinjia Biopharm Co., Ltd | |
 |
2024-04-23 | Upadacitinib
1310726-60-3
|
US $57.00-52.00 / box | 1box | 99% | 5000 box | Hebei Weimiao Import and Export Trade Co., Ltd. | |
 |
2024-04-22 | Upadacitinib
1310726-60-3
|
US $0.00-0.00 / Kg/Bag | 1Kg/Bag | 0.99 | 20 tons | Sinoway Industrial co., ltd. |
-

- Upadacitinib
1310726-60-3
- US $300.00 / kg
- 98%
- Anhui Dexinjia Biopharm Co., Ltd
-

- Upadacitinib
1310726-60-3
- US $57.00-52.00 / box
- 99%
- Hebei Weimiao Import and Export Trade Co., Ltd.
-

- Upadacitinib
1310726-60-3
- US $0.00-0.00 / Kg/Bag
- 0.99
- Sinoway Industrial co., ltd.
1310726-60-3(Upadacitinib)Related Search:
1of4





