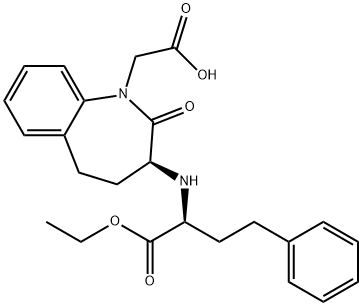
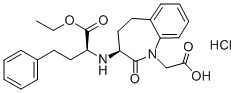
Benazepril
- CAS No.
- 86541-75-5
- Chemical Name:
- Benazepril
- Synonyms
- Briem;Benapril;Cibacene;Cibcen WS;genazepril;BENAZCPRIL;Cibacen WS;Benazepril;Benazapril;BENAZEPRIL MM
- CBNumber:
- CB5348020
- Molecular Formula:
- C24H28N2O5
- Molecular Weight:
- 424.49
- MDL Number:
- MFCD00864466
- MOL File:
- 86541-75-5.mol
| Melting point | 148-149° |
|---|---|
| alpha | D -159° (c = 1.2 in ethanol) |
| Boiling point | 691.2±55.0 °C(Predicted) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO |
| pka | 3.73±0.10(Predicted) |
| BCS Class | 1 |
| CAS DataBase Reference | 86541-75-5(CAS DataBase Reference) |
| FDA UNII | UDM7Q7QWP8 |
| ATC code | C09AA07 |
SAFETY
Risk and Safety Statements
| Safety Statements | 22-24/25 |
|---|
Benazepril price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | B119858 | BenazeprilFreeBase | 86541-75-5 | 2.5g | $1825 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0001603 | BENAZEPRIL 95.00% | 86541-75-5 | 5G | $2607.41 | 2021-12-16 | Buy |
| ApexBio Technology | B1278 | Benazepril | 86541-75-5 | 250mg | $45 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 18510 | Benazepril | 86541-75-5 | 25mg | $1500 | 2021-12-16 | Buy |
| ChemScene | CS-1795 | Benazepril | 86541-75-5 | 500mg | $80 | 2021-12-16 | Buy |
Benazepril Chemical Properties,Uses,Production
Originator
Lotensin,Novartis
Uses
Benazepril Free base is a non-sulfhydryl ACE inhibitor.
Definition
ChEBI: A benzazepine that is benazeprilat in which the carboxy group of the 2-amino-4-phenylbutanoic acid moiety has been converted to the corresponding ethyl ester. It is used (generally as its hydrochloride salt) as a prodrug for the angiotensin-converting enzy e inhibitor benazeprilat in the treatment of hypertension and heart failure.
Manufacturing Process
The synthesis of benzazepril based on a benzazepinone. It started by chlorination of lactam - 1,2,4,5-tetrahydrobenzo[b]azepin-2-one to the dichloro derivative 3,3-dichloro-1,2,4,5-tetrahydrobenzo[b]azepin-2-one. Catalytic reduction removed one of the gem chloro substituents to give 3- chloro-1,2,4,5-tetrahydrobenzo[b]azepin-2-one; the halogen was then displaced with sodium azide to give 3-azido-1,3,4,5- tetrahydrobenzo[b]azepin-2-one. Alkylation of the amide with ethyl bromoacetate in the presence of base yielded the ester (3-azido-2-oxo- 2,3,4,5-tetrahydrobenzo[b]azepin-1-yl)acetic acid ethyl ester. Hydrogenation then converted the azide to an amino group to give 3-amino-2-oxo-2,3,4,5- tetrahydrobenzo[b]azepin-1-yl)acetic acid ethyl ester. It was then resolved by classical salt formation and crystallization. Saponification of the S enantiomer - S-(3-amino-2-oxo-2,3,4,5-tetrahydrobenzo[b]azepin-1-yl)acetic acid ethyl ester with sodium hydroxide afforded (3-amino-2-oxo-2,3,4,5- tetrahydrobenzo[b]azepin-1-yl)acetic acid. Reductive alkylation of it with 2- oxo-4-phenylbutyric acid ethyl ester and sodium cyanoborohydride gave the desired product as 70:30 mixture of diastereoisomers. The isolation of the predominant isomer gave benazepril. The epimerization occurred thermally and therefore required a sufficiently high temperature. The high temperature condition can be achieved by either using a high boiling-point solvent such as xylene or by heating the reaction mixture under pressure to increase its boiling-point temperature. Good results can be achieved in both polar and non-polar solvent systems. For example, both p-xylene and ethylene glycolwater systems are found suitable to conduct this process. The crude product acid 3-[(1-ethoxycarbonyl)-3-phenyl-(1S)-propylamino]-2,3,4,5-tetrahydro-2- oxo-1H-1-benzazepine-1-acetic acid was heated to reflux temperature for 30 hours in p-xylene. The mixture was cooled down to room temperature. Solvent removal resulted in a solid, which was then dried at reduced pressure to give a 98:2 diasteriomeric mixture as determined by HPLC, MP: 287°- 290°C. IR and 1H-NMR spectrum analysis. was confirmed the structure of product.
Therapeutic Function
Antihypertensive
Enzyme inhibitor
This ACE-directed pro-drug (FWfree-acid = 424.50 g/mol; CAS 86541-75-5), also known as 3-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)-2,3,4,5- tetrahydro-2-oxo-(S-(R*,R*))-1H-1-benzazepine-1-acetic acid, Lotensin and CGS 14824A, is rapidly metabolized to its diacid benazeprilat (CGS 14831; FWfree-diacid = 396.44 g/mol), the latter a potent, long-acting inhibitor of peptidyl-dipeptidase A, or angiotensin I-converting enzyme, IC50 = 1.7 nM. Lotensin is used to treat hypertension, congestive heart failure, and chronic renal failure. The antihypertensive effects of benazepril begin as early as 30 min after a single dose, and those effects during consecutive dosing are also sustained for 24 h with a lesser diurnal variation in blood pressure
Benazepril Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jiangxi Huihe Chemical Co., Ltd. | +86-19189228086 +8618858568638 | wuzhouding@huihecrop.com | China | 886 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shandong Hengshannuode Pharmaceutical Technology Co., Ltd. | +8615065888978 | admin@hsnordpharma.com | China | 92 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9553 | 58 |
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 | erin@hbldbiotech.com | China | 879 | 58 |
View Lastest Price from Benazepril manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-14 | Benazepril
86541-75-5
|
US $0.00-0.00 / g | 100g | 99% | 20 tons | Wuhan Han Sheng New Material Technology Co.,Ltd | |
 |
2023-04-19 | Benazepril
86541-75-5
|
US $50.00 / kg | 1kg | 99% | 50 tons | Hebei Duling International Trade Co. LTD | |
 |
2021-11-03 | Benazepril
86541-75-5
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%min | 100kg | WUHAN FORTUNA CHEMICAL CO., LTD |
-

- Benazepril
86541-75-5
- US $0.00-0.00 / g
- 99%
- Wuhan Han Sheng New Material Technology Co.,Ltd
-

- Benazepril
86541-75-5
- US $50.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD
-

- Benazepril
86541-75-5
- US $0.00-0.00 / Kg/Drum
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD