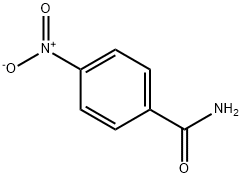
p-Nitrobenzamide
- CAS No.
- 619-80-7
- Chemical Name:
- p-Nitrobenzamide
- Synonyms
- NitrobenzaMide;P-NITROBENZAMIDE;p-nitro-benzamid;4-NITROBENZAMIDE;4-nitro-benzamid;Benzamide,4-nitro-;Benzamide,p-nitro-;TIMTEC-BB SBB008201;Benzamide, p-nitro-;4-Nitrobenzamide>
- CBNumber:
- CB5369841
- Molecular Formula:
- C7H6N2O3
- Molecular Weight:
- 166.13
- MDL Number:
- MFCD00007994
- MOL File:
- 619-80-7.mol
- MSDS File:
- SDS
| Melting point | 199-201 °C(lit.) |
|---|---|
| Boiling point | 294.32°C (rough estimate) |
| Density | 1.4334 (rough estimate) |
| refractive index | 1.5880 (estimate) |
| pka | 15.02±0.50(Predicted) |
| form | powder to crystal |
| color | White to Orange to Green |
| Water Solubility | <0.01 g/100 mL at 18 ºC |
| BRN | 639263 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 619-80-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NIST Chemistry Reference | Benzamide, 4-nitro-(619-80-7) |
| EPA Substance Registry System | p-Nitrobenzamide (619-80-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | T,Xn | |||||||||
| Risk Statements | 23/24/25-22-20/21/22 | |||||||||
| Safety Statements | 27-28-36/37/39-45-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CV5601000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29242990 | |||||||||
| NFPA 704 |
|
p-Nitrobenzamide Chemical Properties,Uses,Production
Chemical Properties
white powder
Uses
4-Nitrobenzamide was used in the preparation of 4-nitrobenziminosulfurane.
Synthesis Reference(s)
Tetrahedron Letters, 36, p. 3469, 1995 DOI: 10.1016/0040-4039(95)00528-K
General Description
White powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A nitrated amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Fire Hazard
Flash point data for p-Nitrobenzamide are not available, however, p-Nitrobenzamide is probably combustible.