Titanous chloride
- CAS No.
- 7705-07-9
- Chemical Name:
- Titanous chloride
- Synonyms
- TiCl3;TITANIUM(III) CHLORIDE;TITANIUM TRICHLORIDE;Titanium chloride (TiCl3);Titanium(iii) chloride, 20% w/w solution in 2n hydrochloric acid;tac121;tac131;TAC 131;TAC 121;TITANOUS CHLORIDE
- CBNumber:
- CB5375851
- Molecular Formula:
- Cl2Ti
- Molecular Weight:
- 118.77
- MDL Number:
- MFCD00011266
- MOL File:
- 7705-07-9.mol
- MSDS File:
- SDS
| Melting point | 440 °C (dec.)(lit.) |
|---|---|
| Boiling point | 100 °C |
| Density | 1.192 g/mL at 25 °C |
| solubility | reacts with H2O |
| form | powder |
| color | Purple |
| Water Solubility | Soluble |
| Sensitive | Air Sensitive |
| Merck | 14,9481 |
| Stability | Stable, but reacts violently with water and is spontaneously flammable in air. |
| InChIKey | ZWYDDDAMNQQZHD-UHFFFAOYSA-L |
| CAS DataBase Reference | 7705-07-9(CAS DataBase Reference) |
| FDA UNII | GVD566MM7K |
| NIST Chemistry Reference | Titanium(iii) chloride(7705-07-9) |
| EPA Substance Registry System | Titanium chloride (TiCl3) (7705-07-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|---|---|
| Signal word | Danger |
| Hazard statements | H290-H314-H335 |
| Precautionary statements | P234-P261-P271-P280-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | C,F |
| Risk Statements | 34-37-35-17-14 |
| Safety Statements | 6-26-30-36/37/39-43-45 |
| RIDADR | UN 3264 8/PG 2 |
| WGK Germany | 3 |
| RTECS | XR1924000 |
| F | 10-23 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 28273990 |
Titanous chloride price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 14010 | Titanium(III) chloride solution 10-15% TiCl3 basis | 7705-07-9 | 250ML | $78.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 14010 | Titanium(III) chloride solution 10-15% TiCl3 basis | 7705-07-9 | 1L | $261 | 2024-03-01 | Buy |
| Sigma-Aldrich | 14010 | Titanium(III) chloride solution 10-15% TiCl3 basis | 7705-07-9 | 6X1L | $1120 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | ING0005620 | TITANIUM TRICHLORIDE 95.00% | 7705-07-9 | 1G | $597.87 | 2021-12-16 | Buy |
Titanous chloride Chemical Properties,Uses,Production
Physical and chemical properties
It appears as deep purple crystal and can be easily subject to deliquescence. Its chemical formula is TiCl3. It has a molecular weight of 154.26. It has a relative density is 2.64, a melting point of 440 °C (decomposition) and the boiling point of 660 °C (106 × 133.322 Pa). It is easily soluble in water and slightly soluble in ethanol with both the solutions exhibiting purple color. Upon heating, the solution will be turned into a blue with further returning back to purple after cooling down. It is unstable and will be faded after standing in the air for a long time with precipitation of metatitanic acid (H2TiO3) precipitate. It can be dissolved in hydrochloric acid and insoluble in ether. Being dissolved in HCl solution will generate titanium tetrachloride tetrahydrate TiCl3 • 4H2O, which is unstable in the air. It will be subject to decomposition under 440 ℃. In the air, it can be oxidized to Ti (Ⅳ) with moisture being able to accelerate the oxidation process, which must be stored in CO2 atmosphere. The purple TiCl3 • 6H2O salt prepared by electrolysis of dilute HCl solution of TiCl4 is more stable.
Preparation: it can be obtained through the interaction between the hydrochloric acid solution of titanium tetrachloride and stannous chloride or alternatively through the hydrogen reduction of the hydrochloric acid solution of titanium tetrachloride.
Applications: (1) it can be used for azo dye analysis titration solution for colorimetric determination of copper, iron, vanadium and so on. (2) In synthetic chemistry, it can be used as the reducing agent of tetravalent chromium and an important component of Ziegler-Natta catalyst [Al (C2H5) 3TiCl3], the catalyst can be used for olefin polymerization.
Image 1 the chemical structure of the titanium trichloride.
Chemical reaction
Titanium trichloride has lively chemical nature, being able to react with a variety of elements or compounds: upon heating and oxidation in O2 gas, it will sometimes undergo combustion and generate TiCl4 and TiO2; in H2 gas stream, it will be reduced to TiCl2; and will react with water vapor at 600 ℃ to generate TiOCl and HCl; In the air, it is easily subject to deliquescence, being soluble in water, alcohol and acid. In acid solution, it can be gradually oxidized in the air by O2, can also be oxidized by Fe3 +, Cr2O72-and VO3-and other oxidants; at high temperature, it can react with HCl to generate TiCl4; it can react with Fe2O3, TiO2 and SiO2 at 600 ℃ to generate TiOCl; Under heating, it can be reduced by alkali metal or alkaline earth metal to generate metal Ti. It is unstable chloride with dissociation at high temperatures and disproportionation reaction generating TiCl2 and TiCl4. For anhydrous titanium trichloride (TiCl3), we can use H2, Na, Mg, Al, and Ti as the reducing agent to make it through reducing TiCl4 under appropriate conditions. The aqueous solution of titanium trichloride can be prepared by dissolving the metal Ti in hydrochloric acid under the protection of an H2 atmosphere or an inert gas.
This information was edited by Xiao Nan from Chemicalbook (2015-08-22).
Four crystal forms of titanium trichloride
Titanium trichloride has four crystal forms and one hexahydrate:
(1) Under high temperature, what obtained through reduction of TiCl4 is alpha type of TiCl3, exhibiting purple sheet structure, belonging to hexagonal system, lattice constant a = 6.122 × 10-8cm, c = 17.52 × 10-8cm. It has a relative density of 2.64. It will undergo decomposition under 440 ° C and has a boiling point of 660 ° C (14.132 × 103Pa).
(2) Reduction of TiCl4 with AlCl3 can give β-TiCl3, a brown powder with fibrous structure. In an inert gas stream and at 250 to 300 ° C, it will be converted to α-type.
(3) Aluminum reduction of TiCl4 will get γ-type TiCl3, a kind of red purple layered crystal.
(4) Grindγ-TiCl3 to obtain δ-type TiCl3, δ-type is purple powder with unknown structure, and has higher catalytic performance than other crystalline TiCl3. It has a melting point of 730 ℃-920 ℃, the relative density of 2.69 and the boiling point of 660 °C (106 × 133.322 Pa). It exhibits purple color after being dissolved in water and slightly dissolved in ethanol with heating leading to blue solution color. The color will go back to become purple after cooling. It will be faded after being stored for a long time under the air, and undergo precipitation of metatitanic acid (H2TiO3) precipitate. It is insoluble in ether. Titanium trichloride is a catalyst for many organic chemical reactions, widely used as the main catalyst for the production of polypropylene. Used as azo dye analysis titration solution, and for colorimetric determination of Cu, Fe and V.
In addition to four different crystal forms, the titanium trichloride also has a hexahydrate (TiCl3 • 6H2O), being further divided into purple stable type and green unstable type due to ligand coordination. It will undergo disproportionation reaction at a temperature of 450 ° C or more to produce titanium dichloride and titanium tetrachloride. It is insoluble benzene, slightly soluble in chloroform and soluble in ethanol. The hexahydrate is a pale purple crystal. It is easy to absorb moisture, being soluble in water. In dry air, it can undergo slow oxidation and decolorization. In wet air, it will be quickly transformed into titanium dichloride hydrate.
Uses
It is mainly used as a strong reducing agent; as the catalyst of Α-olefin polymerization. It can be used for azo dye analysis.
It can be used as the titrant of azo dye analysis and strong reducing agent.
Preparation
Aluminum reduction process: apply an excess of titanium tetrachloride to react with aluminum powder at 136 °C with aluminum chloride being taken as the initiator to produce titanium trichloride and aluminum trichloride. Heat and steam out excess amount titanium tetrachloride for recycling usage. Meanwhile, the aluminum trichloride undergoes sublimation to get finished product of titanium trichloride. Its reaction equation is:
3TiCl4 + Al → 3TiCl3 + A1C13
Explosive properties
it is corrosive
Flammable and Hazardous characteristics
In the case of oxidants, H Hole agent is flammable; in case of cyanide, it will release toxic hydrogen cyanide gas with thermal decomposition generating toxic chloride smoke
Storage and transportation characteristics
Treasury should be ventilated, low-temperature and dry; store it separately from oxidants, cyanide, H Hole agent and alkali.
Extinguishing agent water
water, carbon dioxide, foam
Chemical Properties
purple crystalline solid
Physical properties
Red-violet hexagonal crystals; hygroscopic; density 2.64 g/cm3; decomposes on heating above 425°C; also decomposes in water, evolving heat; soluble in alcohol, acetonitrile and certain amines; insoluble in hydrocarbons and ether.
Uses
Titanium Chloride (≥12% HCl Solution) is ued in the optimized gas chromatography tandem mass spectrometry for 1,1''-sulfonylbis[2-(methylthio)ethane] quantification in human urine
Uses
As powerful reducing agent, Titanous chlorideb can reduces nitrate to ammonia; when boiled with aqueous SO2, sulfur is separated; hence is used as an aqueous solution for estimation of nitro groups, ferric ions, per-salts, etc. Removes stains, etc. (stripper) in laundering.
Preparation
Titanium trichloride may be prepared by reducing titanium tetrachloride with hydrogen at 600°C. The tetrachloride may alternatively be reduced with aluminum, zinc, magnesium, tin, or by electrolysis.
General Description
A dark violet crystalline solid. Denser than water. Contact may burn skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
Air & Water Reactions
Pyrophoric, very reactive with water and moisture in air produces hydrochloric acid, [Merck 11th ed. 1989]. Ignites spontaneously on contact with air; decomposed by water and water vapor forming HCl. [Handling Chemcials Safely 1980. p. 905].
Reactivity Profile
Acidic salts, such as TITANIUM TRICHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. Ethylene can polymerize at low pressure if catalyzed by titanium halides. (Sundaram, K. M, M. M. Shreehan, E. F. Olszewski. thylene. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2001.)
Hazard
Fire risk in the presence of oxidizing materials. Irritant to skin and tissue; open container only in oxygen-free or inert atmosphere.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Flammability and Explosibility
Pyrophoric
Safety Profile
A corrosive irritant to skin, eyes, and mucous membranes. A severe corrosive because it liberates heat and hydrochloric acid upon contact with moisture. If spilled on slun, wipe off with dry cloth before applying water. May ignite spontaneously in air. Flammable when exposed to heat or flame. Reacts violently with K, HF. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of Cl-. See also TITANIUM COMPOUNDS.
Purification Methods
It is a brown purple powder that is very reactive to H2O and pyrophoric when dry. It should be manipulated in a dry box. It is soluble in CH2Cl2 and tetrahydrofuran, and is used as a M solution in these solvents in the ratio of 2:1, and stored under N2. It is a powerful reducing agent. [Ingraham et al. Inorg Synth VI 52 1960.]
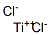
Titanous chloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Qingdao kaimoshi biochemical technology co., ltd. | +86-0571-87191913 +86-19957014543 | info@kaimosi.com | CHINA | 144 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9309 | 58 |
View Lastest Price from Titanous chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-01-28 | Titanium chloride(TiCl3)
7705-07-9
|
US $0.00 / Kg/Drum | 10Kg/Drum | 99.0% min | 100 Ton/Tons per Month | Qingdao kaimoshi biochemical technology co., ltd. | |
 |
2019-07-14 | Titanous chloride
7705-7-9
|
US $1.00 / KG | 1KG | 995 | 25kg | Career Henan Chemical Co |
-

- Titanium chloride(TiCl3)
7705-07-9
- US $0.00 / Kg/Drum
- 99.0% min
- Qingdao kaimoshi biochemical technology co., ltd.
-

- Titanous chloride
7705-7-9
- US $1.00 / KG
- 995
- Career Henan Chemical Co