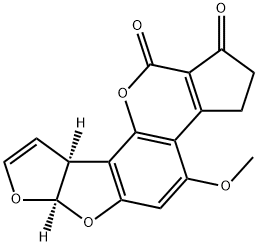
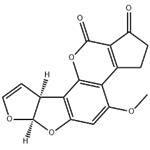
AFLATOXIN B1
- CAS No.
- 1162-65-8
- Chemical Name:
- AFLATOXIN B1
- Synonyms
- afb1;AFLATOXIN B;AFBI;AflatoxinB1(form1);aflatoxin b1solution;Aflatoxin B1, 98%, from Aspergillus flavus;Aflatoxin B1 Solution in Acetonitrile, 100μg/mL;HSDB 3453;NSC 529592;alflatoxin
- CBNumber:
- CB5389650
- Molecular Formula:
- C17H12O6
- Molecular Weight:
- 312.27
- MDL Number:
- MFCD00869647
- MOL File:
- 1162-65-8.mol
| Melting point | 268-269 °C |
|---|---|
| alpha | D -558° (c = 0.1 in CHCl3); D -480° (c = 0.1 in DMF) |
| Boiling point | 372.21°C (rough estimate) |
| Density | 1.2810 (rough estimate) |
| refractive index | 1.4800 (estimate) |
| Flash point | 2 °C |
| storage temp. | 2-8°C |
| solubility | DMF: 20 mg/ml; DMF:PBS(pH 7.2)(1:1): 0.5 mg/ml; DMSO: 12 mg/ml |
| form | White to yellow powder. |
| Water Solubility | 15mg/L(temperature not stated) |
| Merck | 13,180 |
| BRN | 1269174 |
| Stability | Stable. Incompatible with strong oxidizing agents. May be light or air sensitive. |
| InChIKey | OQIQSTLJSLGHID-WNWIJWBNSA-N |
| LogP | 2.039 (est) |
| FDA UNII | 9N2N2Y55MH |
| EPA Substance Registry System | Aflatoxin B1 (1162-65-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H340-H350-H361 | |||||||||
| Precautionary statements | P201-P262-P280-P301+P310+P330-P302+P352+P310-P304+P340+P310 | |||||||||
| Hazard Codes | T+,T,Xn,F | |||||||||
| Risk Statements | 45-46-26/27/28-36-20/21/22-11-65-48/23/24/25-36/38-39/23/24/25-23/24/25 | |||||||||
| Safety Statements | 53-45-36-26-16-24-7-62-36/37-28 | |||||||||
| RIDADR | UN 3462 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GY1925000 | |||||||||
| F | 10 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29322090 | |||||||||
| Toxicity | LD50 orally in day old duckling: 18.2 mg/50 gm body wt (Carnaghan); i.p. in newborn mice: 9.50 mg/kg body wt (Büchi) | |||||||||
| NFPA 704 |
|
AFLATOXIN B1 price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A6636 | Aflatoxin B1 from Aspergillus flavus from Aspergillus flavus | 1162-65-8 | 1mg | $75.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | CRM46323 | Aflatoxin B1 solution certified reference material, 3?μg/mL in benzene:acetonitrile (98:2), ampule of 1?mL | 1162-65-8 | 1mL | $86.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | CRM44647 | Aflatoxin B1 solution certified reference material, 20?μg/mL in methanol, ampule of 1?mL | 1162-65-8 | 1mL | $171 | 2024-03-01 | Buy |
| Sigma-Aldrich | 34029 | Aflatoxin B1 solution 2?μg/mL in acetonitrile, analytical standard | 1162-65-8 | 2ml | $339 | 2024-03-01 | Buy |
| Cayman Chemical | 11293 | Aflatoxin B1 ≥98% | 1162-65-8 | 1mg | $46 | 2024-03-01 | Buy |
AFLATOXIN B1 Chemical Properties,Uses,Production
Overview
Aflatoxin B1 (AFB1) is primarily produced by the common fungus Aspergillus flavus and A. parasiticus, which is a closely related species. Among the aflatoxins, Aflatoxin B1 is the most hepatocarcinogenic and hepatotoxic and occurs as a contaminant in a variety of foods. AFB1 is a potent mutagen, toxin, and carcinogen and can be traced in the etiology of hepatocarcinoma. Although AFB1 commonly affects the life, studies have revealed that it is also produced in the colons, kidneys, and lungs of rodents. It is noteworthy that AFB1 is typically nonreactive until it is ingested and converted by the enzymes in the liver to a reactive intermediate known as AFB1 8,9 –oxide.
Exposure to AFB1
AFB1 is the most toxic and occurs at the highest levels among the different aflatoxins. The main source of exposure to AFB1 is contaminated food including corn, peanuts, fig, soybeans, nuts, and cheese. Typically, AFB1 production is normally increased in hot and humid environments or climates. As such, AFB1 production is common in South America, South East Asia, and Sub-Saharan Africa. Occupational exposure has been proved to occur in individuals working in pig and poultry production.
Pathology
AFB1 can enter the body of a human being through the skin, especially in hot or humid environmental conditions. The most vulnerable body organ to AFB1 is the liver. Pathological lesions that are linked to AFB1 in animals include weight loss of the liver, hepatic carcinoma, and vacuolation of hepatocytes. Others include necrosis, hepatic cells, fatty infiltration, fibrosis, hemorrhage, bile duct hyperplasia, and regeneration of nodules.
Mechanism of Action of AFB1 in developing Hepatocellular Carcinoma (HCC)
Food mutagens are classified as non-genotoxic and genotoxic agents depending on their mechanism of action in triggering cancer. AFB1 is a genotoxic hepatocarcinogen agent that leads to genetic alterations with the mode of action beginning at the DNA level. It causes cancer by inducing DNA adducts, thereby leading to genetic alterations in target cells that then lead to breakage of the DNA strand, oxidative damage and DNA base damage that may cause cancer. Notably, DNA adducts are generated by the chemical modification of the bases in amino acids in proteins or DNA by toxic carcinogenic chemicals. Studies have revealed the p53 gene causes about 50% of cancer in human beings and that mutations affecting the gene are varied by their position and nature. For instance, mutations like the transversion in codon 249 that leads to arginine to serine substitution are present in half of HCCs.
AFB1 target for metabolism is the liver where its mechanism of action is started. After food is ingested, AFB1 is normally metabolized by cytochrome-P450 enzymes that are reactive to hydroxylated to AFQ1 and AFM1, genotoxic intermediates and demethylated to AFP1 to become less harmful as compared to AFB1. Cytochrome-P450 has to biotransform AFB1 before can exact its hepatocarcinogenic effect. It is noteworthy that this process usually results in the production of AFBO, which is a reactive intermediate chemical compound. AFBO, a genotoxic compound, will then bind to the liver cell DNA as a result, leading to the formation of DNA adducts. In case there is no repair before the replication of DNA, the DNA adducts will interact with the guanine base of the DNA, leading to mutational impacts in the p53 tumour suppressor gene, thus resulting in hepatocarcinogenesis. Inhibition of apoptosis and stimulation of liver cell growth may occur due to the expression of mutated R249p53.
Food Regulation
Dietary exposure to mutagens and carcinogens depends on distinct ingesting patterns that normally change with the availability of food, age, and lifestyle. AFB1 usually contaminates animal and human foods during cultivation, crop growth, harvesting, and storage. It is noteworthy that prevention of hepatocellular carcinogenesis and hepatotoxicity of AFB1 requires durable interventions such as the implementation of comprehensive food programs. The responses should touch on both local farmers and market vendors to minimize or prevent long-term exposure to AFB1, thereby decreasing HCC incidence rates.
Pre-harvest interventions include cultivating crops that are resistant to aflatoxin biosynthesis and fungal infection as well as spraying of fungicides and insecticides. Application of low-technology interventions in the reduction of AFB1 ¬after harvesting has been suggested such as drying on mats, hand sorting, and avoids the use of plastics.
Health Hazard Information
Numerous studies have been conducted regarding the toxicity of AFB1, which might be acute or toxic.
AFB1 poisoning is referred to as aflatoxicosis, which may occur because of consumption of contaminated food. AFB1 can cause chronic reproduction affects when swine consumer high doses. Chronic symptoms on AFB1 toxicity include reduced growth rate and weight, decreased appetite, poorer carcass quality, lower milk production, immunity to the vaccine, and lower disease resistance.
Notable Exposures
One of the noteworthy exposure to AFB1 ¬is the 1960’s death of over 100, 000 turkeys in England. In Kenya, 12 patients died of acute AFB1 ¬poisoning after consuming contaminated maize in 1981.
Chemical Properties
The aflatoxins are a group of molds produced by the fungus Aspergillus flavus. They are natural contaminants of fruits, vegetables, and grains. They are also described as a series of condensed ring heterocyclic compounds. They form colorless to pale yellow crystals. Practically insoluble in water.
Uses
Aflatoxin B1 is the major analogue of a family of bisfuranocoumarin mycotoxins produced by Aspergillus flavus and related species. Aflatoxin B1 exhibits a distinctive UV spectrum and blue fluorescence. Aflatoxins are among the most potent mycotoxins known but are in fact "pre-toxins", requiring metabolic activation to the toxic principle. Aflatoxins are found widely in nature in trace amounts, particularly in grains and nuts. The toxicity of these metabolites was first recognised in the 1950s and their structures elucidated in 1963. Aflatoxins have been extensively reviewed.
Uses
Aflatoxin B1 is a carcinogenic compound that induces transversion of G to T at codon 249 of the p53 tumor suppressor gene. Aflatoxin B1 may be used as an internal standard when testing for aflatoxin contamination in food products.
Definition
ChEBI: An aflatoxin having a tetrahydrocyclopenta[c]furo[3',2':4,5]furo[2,3-h]chromene skeleton with oxygen functionality at positions 1, 4 and 11.
General Description
Colorless to pale yellow crystals or white powder. Exhibits blue fluorescence.
Air & Water Reactions
Sensitive to exposure to air and light. Water insoluble.
Reactivity Profile
AFLATOXIN B1 is incompatible with strong oxidizing agents, strong acids and strong bases.
Fire Hazard
Flash point data for AFLATOXIN B1 are not available; however, AFLATOXIN B1 is probably combustible.
Biochem/physiol Actions
Aflatoxin B1 is a carcinogenic compound produced by Aspergillus flavus, a common soil fungus, that induces transversion of G to T at codon 249 of the p53 tumor suppressor gene. Aflatoxin B1 is a food contaminant and a hepatocarcinogen. Aflatoxin is biotransformed to genotoxic intermediates by P450 Phase I enzymes, mainly CYP3A4 via aflatoxin B1 3-hydroxylation. Detoxification depends on Phase II enzymes, such as Glutathione S-Transferase and AFB(1)-aldehyde reductase (AFAR). Aflatoxin B1 is a CYP1A2, CYP2A6, CYP2D6, and CYP3A family substrate.
Safety Profile
Confirmed human carcinogen with experimental tumorigenic, neoplas tigenic, and carcinogenic data. Acute poison by ingestion, intraperitoneal, and possibly other routes. Experimental teratogenic and reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke. See also various aflatoxins.
Potential Exposure
Aflatoxins are a group of toxic metabolites produced by certain types of fungi. Aflatoxins are not commercially manufactured; they are naturally occurring contaminants that are formed by fungi on food during conditions of high temperatures and high humidity. Most human exposure to aflatoxins occurs through ingestion of contaminated food. The estimated amount of aflatoxins that Americans consume daily is estimated to be 0.15 0.50 μg. Grains, peanuts, tree nuts, and cottonseed meal are among the more common foods on which these fungi grow. Meat, eggs, milk, and other edible products from animals that consume aflatoxincontaminated feed may also contain aflatoxins. Aflatoxins can also be breathed in
Metabolic pathway
Aflatoxin B1 can be activated via the monooxygenase reaction which then reacts with the N7 atom of B-DNA guanine. Conjugation of aflatoxin B1 8,9-epoxide is an important detoxification route. Although aflatoxin B1 8,9-epoxide can be hydrolyzed to the diol by epoxide hydrolase, the diol product is toxic, since it reacts readily with proteins by Schiff base formation or binds to DNA. Glutathione conjugation prevents toxicity of both the epoxide and its hydrolysis product. The aflatoxin glutathione conjugate is subsequently excreted from the hepatocyte into bile as a major biliary metabolite.
Shipping
UN3172 Toxins, extracted from living sources, solid or liquid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Use of oxidizing agents, such as hydrogen peroxide or 5% sodium hypochlorite bleach. Acids and bases may also be used.
AFLATOXIN B1 Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7377 | 58 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9352 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | scglp@glp-china.com | CHINA | 1824 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | sales@pioneerbiotech.com | China | 3000 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | contact@fuxinpharm.com | China | 10297 | 58 |
View Lastest Price from AFLATOXIN B1 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-29 | AFLATOXIN B1
1162-65-8
|
US $8.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-06-27 | AFLATOXIN B1
1162-65-8
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-11-05 | AFLATOXIN B1
1162-65-8
|
US $10.00-200.00 / G | 100G | 99.99% | 20MT | Hebei Ningnan Trade Co. LTD |
-

- AFLATOXIN B1
1162-65-8
- US $8.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- AFLATOXIN B1
1162-65-8
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- AFLATOXIN B1
1162-65-8
- US $10.00-200.00 / G
- 99.99%
- Hebei Ningnan Trade Co. LTD