Arbidol hydrochloride
- CAS No.
- 131707-23-8
- Chemical Name:
- Arbidol hydrochloride
- Synonyms
- Abidol;Doxetine;ARBIDOL HCL;Abiduoer.HCI;Arbidole HCl;Umifenovir HCl;yansuanabiduoer;Soluble Arbidol;Arbidol hydrochlorid;ARBIDOL HYDROCHLORIDE
- CBNumber:
- CB5438596
- Molecular Formula:
- C22H26BrClN2O3S
- Molecular Weight:
- 513.88
- MDL Number:
- MFCD00344795
- MOL File:
- 131707-23-8.mol
- MSDS File:
- SDS
| Melting point | 133-137?C |
|---|---|
| storage temp. | -20°C |
| solubility | DMSO: soluble20mg/mL protein, clear |
| form | powder |
| color | white to beige |
| Stability | Hygroscopic |
| InChI | InChI=1S/C22H25BrN2O3S.ClH/c1-5-28-22(27)20-18(13-29-14-9-7-6-8-10-14)25(4)17-11-16(23)21(26)15(19(17)20)12-24(2)3;/h6-11,26H,5,12-13H2,1-4H3;1H |
| InChIKey | OMZHXQXQJGCSKN-UHFFFAOYSA-N |
| SMILES | C(C1=C(N(C)C2=CC(Br)=C(O)C(CN(C)C)=C12)CSC1C=CC=CC=1)(=O)OCC.Cl |
| CAS DataBase Reference | 131707-23-8(CAS DataBase Reference) |
| FDA UNII | 8CXV4OU367 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P305+P351+P338-P332+P313-P337+P313 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/38 | |||||||||
| Safety Statements | 26 | |||||||||
| WGK Germany | 3 | |||||||||
| Toxicity | guinea pig,LD50,oral,> 3gm/kg (3000mg/kg),Drugs of the Future. Vol. 17, Pg. 1079, 1992. | |||||||||
| NFPA 704 |
|
Arbidol hydrochloride price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0860 | Arbidol hydrochloride ≥98% (HPLC) | 131707-23-8 | 10mg | $76.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML0860 | Arbidol hydrochloride ≥98% (HPLC) | 131707-23-8 | 50mg | $298 | 2024-03-01 | Buy |
| Cayman Chemical | 16933 | Arbidol (hydrochloride) ≥98% | 131707-23-8 | 5mg | $49 | 2024-03-01 | Buy |
| Cayman Chemical | 16933 | Arbidol (hydrochloride) ≥98% | 131707-23-8 | 10mg | $87 | 2024-03-01 | Buy |
| Cayman Chemical | 16933 | Arbidol (hydrochloride) ≥98% | 131707-23-8 | 50mg | $233 | 2024-03-01 | Buy |
Arbidol hydrochloride Chemical Properties,Uses,Production
Double-effect anti-influenza virus drugs
Arbidol is a kind of double-effect anti-influenza various drug developed by the research center of the chemical drug (former Soviet) and can simultaneously fight against the influenza caused by both influenza virus A and B while may also have antiviral activity against other kinds of certain respiratory viral infections. It has been first approved for entering into market in 1993 in Russia for the control of influenza s well as other acute respiratory viral infections.
According to the different antigen, influenza viruses are divided into A, B, C three types. Because the variation of the influenza virus is relative fast, so humans can’t get long-lasting immunity. For the treatment of influenza, clinically people majorly focus on treating symptoms and preventing secondary infection while being lack of therapeutic agent for targeting the cause of disease. There are three major research directions at home and abroad: vaccines, interferon and anti-viral chemotherapy. The volatility of an influenza virus antigen and the multiplicity of the acute respiratory viral infections have imposed insurmountable obstacles for the vaccines development. Moreover, interferon, due to various reasons including source, dosage and side effects, has been limited in the application of the prevention and treatment of influenza. Therefore, the development of antiviral chemotherapy is an important direction for prevention and treatment of influenza.
Currently, clinical used antiviral chemotherapy drugs only include three kinds: ribavirin, amantadine and rimantadine. Among them, ribavirin, although has antiviral activity on influenza virus A and B at high doses but have teratogenic and mutagenic toxicity like nucleoside drugs and can cause anemia and immune suppression while amantadine and rimantadine are only effective in treating influenza A and is easy to cause central nervous system toxicity, thereby reducing the clinical value.
Arbidol hydrochloride, compared with other antiviral drugs, has the following eight characteristics:
1, it is capable of killing both influenza virus A and B at the same time.
2, it can prevent the viral integration and replication.
3, it can induce the generation of interferon and significantly improve the immunity of human body.
4, it can significantly reduce the flu symptoms and shorten the duration of flu.
5, it can prevent the occurrence and post-development of influenza syndrome.
6, administration of it can effective prevent the influenza upon the pandemic of flu.
7, in vitro tests have demonstrated that it is effective in treating both SARS virus and avian influenza virus.
8, it is convenient for oral administration with high safety and good compliance.
Pharmacological effects
Arbidol hydrochloride having carboxylic acid ester structure and may be a carrier pro-drug. Structure of this drug is mostly lipophilic. As the hydrolysis substrate, it is subject to simple in vivo hydrolysis and the carrier is removed. It then forms active compound and accumulates in the cells, ultimately playing its pharmacological role. The mechanism of action of Arbidol hydrochloride is through activating Arbidol hydrochloride 2,5-oligoadenylate synthetase and specifically preventing the contact, adhesion and fusion between influenza virus envelope with the host cell membrane, thereby blocking the replication of influenza virus, and can penetrate the nucleus to directly inhibit the viral RNA and DNA synthesis; on the other hand, it has immunomodulatory, interferon-induction and antioxidant properties. It is able to stimulate the host cells to produce interferon and stimulate humoral response and phagocytosis of macrophages, causing cellular and humoral immunity, suggesting that its anti-viral effect is closely related to improving the immune function. Anti-infection mechanisms: acidic condition (pH 5.0) is the necessary condition for influenza virus-mediated fusion with a slight increase in pH being capable of preventing the fusion process. Water soluble arbidol is a weak alkaline drug and is capable of increasing the pH of cytoplasm of the host cells so that the virus can not complete the process of membrane fusion for release of the viral genome which effectively suppresses the acute and chronic infection caused by HCV belonging to the Orthomyxoviridae.
Toxicological effects
Arbidol hydrochloride has a relative less toxicity. According to the report of foreign literature, rats and guinea pig being subject to single oral administration of 2000 mg/kg could be well tolerated with the LD50 being larger than 3000mg/kg. Mouse: oral administration-LD50: 340mg/kg. Chronic toxicity test: Rats: 100~125mg/kg, dogs: 25 mg/kg and can subject to oral administration for 6 months without occurrence of pathological changes. It is also safe for long-term administration of drug on rabbits and guinea pigs. Both in vivo and in vitro experiments have shown that it has no mutagenic effect. Reproductive toxicity studies in rats have found no teratogenic effects. LD50> 4g/kg; administration of the recommended dose of drugs won’t cause any adverse effects on the human body.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Clinical application
Arbidol(131707-23-8) is suitable for the prevention and treatment of the following infection diseases in adults and children such as influenza A, influenza B, acute viral respiratory infections, severe acute respiratory syndrome including complicated bronchitis and pneumonia. Administration of arbidol hydrochloride during the early stage after the onset of influenza can significantly reduce the duration of the disease, reduce the severity of symptoms and have an excellent efficacy in alleviating a series of symptoms for influenza patients such as cough, headache, fever, chills, sweating, sore throat, muscle aches and fatigue symptoms. Moreover, it also has good security and is suitable for clinical application. Usage and dosage: 100 mg/tablets, oral administration, for children over the age of 13 as well as each adult: have two tablet per time while taking one tablet per time for children from age 6 to 12; for children from 2 to 6 years old, administer orally of 50mg. Treatment: 3 times per day and continuously take 3~5d. Prevention: take once every three days during the influenza epidemics and continue the administration for three weeks; patients at high risk of contact should have 1 time per day and continue for two weeks.
There has been more than 10 years history of experience in the clinical application of arbidol in Russia, Ukraine and other countries. Arbidol has been already recommended by the Russian State Pharmacopoeia Commission to adults and children to be as the treatment and prevention for influenza A virus and influenza B virus. Leneva et al reported the clinical study on 500 cases of patients of acute viral respiratory infections who had received the treatment of arbidol, the efficiency was as high as 84.8%, Arbidol can significantly shorten the during period of fever, poisoning and inflammation of the respiratory tract, alleviate the symptom and shorten the time for the viral antigens to be released from nasopharyngeal with excellent clinical effect. Wang Mengzhao et al conducted a randomized, double-blind, placebo-controlled clinical study in the evaluation of efficacy as well as safety of this product in the treatment of the naturally acquired influenza. They select 232 cases of patients of 18 to 65 years, with flu-like symptoms for 36h and body temperature being above 37.8 ℃. 125 cases of patients had administrated the drug subjects in accordance with the provisions of medication and had also completed all visits as well as been proved to be suffering influenza virus infection by laboratory tests. They were randomly divided into two groups. The experimental group contains 59 cases of PPi cases while the control group contains 66 patients. The patients in the experimental group took 0.2 g of the product for three times per day and continuously administered for 5 days. The results demonstrated that the remission rate in the experimental group is higher than that in the control group. 232 cases were available for safety analysis with the experimental group containing 113 cases and the control group containing 119 cases.
There were 20 cases of adverse events that may be related to the drug, of which, the experimental group contained 7 patients (6.19%) while the control group contained 13 cases (10.90%) with no significant statistic difference between these two groups (P> 0.05), demonstrating that the goods, when administered during the early stage after the onset of influenza, can shorten the duration of the disease and alleviate the symptoms.
Side effects
The incidence of adverse events was about 6.2% and mainly manifested as nausea, diarrhea, dizziness and elevated level serum transaminases. Youqi Chen et al have conducted pharmacological studies on the safety of the arbidol and the results demonstrated the clinical application of arbidol for the prevention and treatment of influenza, severe respiratory infection, complicated by bronchitis and pneumonia. The clinical dosage: dosage for prevention purpose: 100~200 mg/d; dosage for therapeutic purpose: 800 mg/d. The experimental results have showed that when mice was subject to gavage administration of arbidol 10,50, 250 mg/kg, except 250mg/kg (20 times higher than the clinical dose) had a synergistic effect with sodium pentobarbital for causing sleeping, the general behavior, righting reflex, locomotor activity, sensation and memory function of mice were all not affected. Gavage administration of arbidol for 10, 30, 90 mg/kg had no significant effect on the body temperature of domestic rabbits; Gavage administration of arbidol for 20 mg/kg and 80 mg/kg had no significant effect on the breathing frequency and amplitude, systolic blood pressure & diastolic blood pressure, heart rate, heart rate, ECG QRS wave, T wave, ST segment and PR interval; Arbidol hydrochloride has no obvious effect on the central nervous system, respiratory and cardiovascular systems.
Description
Umifenovir is a broad-spectrum antiviral agent. It inhibits the replication of H5N1 influenza (IC50 = 30 μg/ml) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro (IC50 = 3.537 μM). Umifenovir inhibits entry of hepatitis C virus (HCV) pseudoparticles in Huh7 cells (IC50 = 6 μg/ml) and inhibits Zika virus protein synthesis in Vero cells. In vivo, umifenovir (45 mg/kg) reduces the formation of influenza-induced lung lesions in ferrets.
Chemical Properties
Off White to Pale Yellow Solid
Uses
Arbidol hydrochloride (ARB) is a small monocular compound. It is used to prevent severe pneumonia and virus-associated cytokine dysregulation induced by influenza viruses (IFV). ARB also possesses some immunomodulatory properties, including the effects of interferon induction and macrophage activation.
Arbidol is a broad-spectrum antiviral that has demonstrated activity against a number of enveloped and non-enveloped viruses, and is used clinically to treat influenza. Arbidol inhibits viral entry into host cell and stimulates immune response. Arbidol inhibits fusion between the viral capsid and the cell membrane of the target cell, thus preventing viral entry.
Uses
Arbidol is an antiviral treatment for influenza infection.
Biochem/physiol Actions
Arbidol hydrochloride (ARB) is a small monocular compound. It is used to prevent severe pneumonia and virus-associated cytokine dysregulation induced by influenza viruses (IFV). ARB also possesses some immunomodulatory properties, including the effects of interferon induction and macrophage activation.
storage
Store at -20°C
References
1) Blasing?et al.?(2014), Arbidol as a broad-spectrum antiviral: an update; Antiviral Res,?107?84 2) Boriskin?et al. (2008) Arbidol: a broad-spectrum antiviral compound that blocks viral fusion; Curr. Med. Chem.,?15?997 3) Brooks?et al.?(2012) Antiviral activity of Arbidol, a broad-spectrum drug for use against respiratory viruses, varies according to test conditions; J. Med. Virol.,?84?170 4) Leneva?et al.?(2009)?Characteristics of Arbidol-resistant mutants of influenza virus: Implications for the mechanism of anti-influenza action of arbidol; Antiviral Res.,?81?132 5) Wang?et al.?(2020)?The anti-influenza drug, arbidol, is an efficient inhibitor of SARS-CoV-2 in vitro; Cell Discov.,?6?28 6) Vankadari?(2020)?Arbidol: A Potential Antiviral Drug for the Treatment of SARS-CoV-2 by Blocking Trimerization of the Spike Glycoprotein; Int. J. Antimicrob. Agents, 56 105998

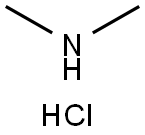
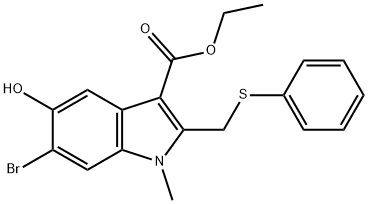
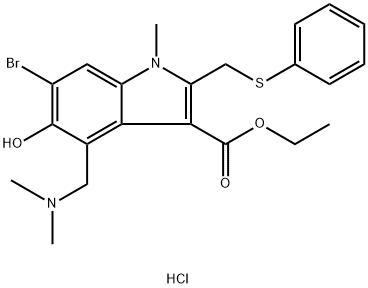
Arbidol hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Guangzhou TongYi biochemistry technology Co.,LTD | +8613073028829 | mack@tongyon.com | China | 2996 | 58 |
| Jinan Jianfeng Chemical Co., Ltd | 0531-88110457; +8615562555968 | info@pharmachemm.com | China | 246 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-27-59207850 +86-13986145403 | info@fortunachem.com | China | 5986 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9427 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Nanjing Fred Technology Co., Ltd | +86-25-84696168 +86-15380713688 | Austin@fredbio.com | China | 2449 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
Related articles
- Different formulations and pharmacokinetics of Arbidol hydrochloride
- Arbidol hydrochloride is a broad-spectrum antiviral agent that targets a wide range of enveloped and non-enveloped viruses.
- Dec 13,2023
- Uses of Arbidol hydrochloride
- Arbidol hydrochloride is licensed in Russia and China mainly for treatment of influenza infection and other respiratory viral ....
- May 9,2022
- Side effects of Arbidol Hydrochloride
- Arbidol hydrochloride has the effect of an immunomodulator and also has special antiviral activity against influenza A and inf....
- Dec 10,2021
View Lastest Price from Arbidol hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Arbidol hydrochloride
131707-23-8
|
US $0.00-0.00 / Kg/Bag | 0.1Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-07 | Arbidol HCl
131707-23-8
|
US $0.00-0.00 / g | 1g | 99.9% | 20 tons | airuikechemical co., ltd. | |
 |
2024-03-16 | Arbidol hydrochloride
131707-23-8
|
US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD |
-

- Arbidol hydrochloride
131707-23-8
- US $0.00-0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- Arbidol HCl
131707-23-8
- US $0.00-0.00 / g
- 99.9%
- airuikechemical co., ltd.
-

- Arbidol hydrochloride
131707-23-8
- US $0.00 / KG
- 98%+
- WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD
131707-23-8(Arbidol hydrochloride)Related Search:
1of4





