Diethylamine
- CAS No.
- 109-89-7
- Chemical Name:
- Diethylamine
- Synonyms
- DEA;Diethylamin;detn;Dietilamina;N-ETHYLETHANAMINE;DIETHYLAMINE FOR SYNTHESIS;ai3-24215;(C2H5)2NH;Diethamine;Diaethylamin
- CBNumber:
- CB5447259
- Molecular Formula:
- C4H11N
Lewis structure
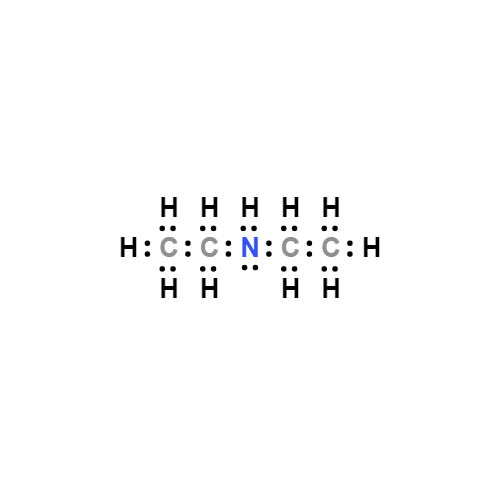
- Molecular Weight:
- 73.14
- MDL Number:
- MFCD00009032
- MOL File:
- 109-89-7.mol
- MSDS File:
- SDS
| Melting point | -50 °C (lit.) |
|---|---|
| Boiling point | 55 °C (lit.) |
| Density | 0.707 g/mL at 25 °C (lit.) |
| vapor density | 2.5 (vs air) |
| vapor pressure | 14.14 psi ( 55 °C) |
| refractive index |
n |
| Flash point | −20 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble1M at 20°C, clear, colorless |
| pka | 11.02(at 40℃) |
| form | Liquid |
| color | Clear colorless |
| Odor | Ammoniacal; sharp, fishy. |
| Relative polarity | 0.145 |
| PH | 13 (100g/l, H2O, 20℃) |
| PH Range | Strong alkaline |
| Odor Threshold | 0.048ppm |
| Evaporation Rate | 16.9 |
| explosive limit | 2.0-11.8%(V) |
| Odor Type | ammoniacal |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| Merck | 14,3111 |
| BRN | 605268 |
| Henry's Law Constant | 2.56(x 10-5 atm?m3/mol) at 25 °C (Christie and Crisp, 1967) |
| Exposure limits | NIOSH REL: TWA 10 ppm (30 mg/m3), STEL 25 ppm (75 mg/m3), IDLH 200 ppm; OSHA PEL: TWA 25 ppm; ACGIH TLV: TWA 5 ppm, STEL 15 ppm (adopted). |
| Dielectric constant | 3.7(20℃) |
| Stability | Stable. Highly flammable. Incompatible with strong oxidizing agents. |
| InChIKey | HPNMFZURTQLUMO-UHFFFAOYSA-N |
| LogP | 0.58 at 20℃ |
| Indirect Additives used in Food Contact Substances | DIETHYLAMINE |
| FDA 21 CFR | 175.105; 177.2600 |
| CAS DataBase Reference | 109-89-7(CAS DataBase Reference) |
| EWG's Food Scores | 2-6 |
| FDA UNII | B035PIS86W |
| NIST Chemistry Reference | Ethanamine, N-ethyl-(109-89-7) |
| EPA Substance Registry System | Diethylamine (109-89-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302+H332-H311-H314-H335 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | F,C | |||||||||
| Risk Statements | 11-20/21/22-35 | |||||||||
| Safety Statements | 16-26-29-36/37/39-45-3 | |||||||||
| RIDADR | UN 1154 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | HZ8750000 | |||||||||
| F | 10-23 | |||||||||
| Autoignition Temperature | 594 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29211990 | |||||||||
| Toxicity | LD50 orally in rats: 540 mg/kg (Smyth) | |||||||||
| IDLA | 200 ppm | |||||||||
| NFPA 704 |
|
Diethylamine price More Price(35)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.03010 | Diethylamine for synthesis | 109-89-7 | 100mL | $28.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03010 | Diethylamine for synthesis | 109-89-7 | 500mL | $33.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03010 | Diethylamine for synthesis | 109-89-7 | 1L | $36.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03010 | Diethylamine for synthesis | 109-89-7 | 2.5L | $70.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | DX1045 | Diethylamine | 109-89-7 | 500mL | $209 | 2024-03-01 | Buy |
Diethylamine Chemical Properties,Uses,Production
Description
Diethylamine is a colourless, strongly alkaline, fish odour liquid, and highly inflammable. It has an ammonia-like odour and is completely soluble in water. On burning, diethylamine releases ammonia, carbon monoxide, carbon dioxide, and nitrogen oxides. Diethylamine is used in the production of pesticides. It is used in a mixture for the production of DEET which goes into the repellents that are found readily in supermarkets for general use.
Physical properties
Colorless liquid with a fishy, ammonia-like odor. Experimentally determined detection and recognition odor threshold concentrations were 60 μg/m3 (20 ppbv) and 180 μg/m3 (60 ppbv), respectively (Hellman and Small, 1974). Diethylamine is a very strong base in aqueous solution (pKb = 3.0). Its chemistry is governed by the unshared electron pair on the nitrogen, thus it tends to react with acids to form salts.
Occurrence
Diethylamine occurs in low concentrations in food and other biological materials. Concentrations (in p.p.m.) in fresh products include: spinach (15), apples (3), butterbeans (2.4), shelled peas (0.1), bean salad (1.5) and red cabbage (2.4) (HSDB 1989). Pickled vegetables contain 0-3.2 p.p.m. diethylamine while concentrations (in p.p.m.) in other materials include herring (0-5.2), barley (5.7), hops (3.1), boiled beef (2), tobacco leaf (0.1-35) and cigarette smoke concentrate (0-0.4). Interest in the occurrence of diethylamine in foods arises in part because of its possible formation of a carcinogenic N-nitroso derivative (Neurath et al 1977). Diethylamine has been reported in the exhaust from a gasoline engine (Hampton et al 1982).
Uses
Diethylamine is manufactured by heating ethyl chloride and alcoholic ammonia under pressure or by hydrogenation of aziridines in the presence of catalysts. DEA is used as a solvent, as a rubber accelerator, in the organic synthesis of resins, dyes, pesticides, and pharmaceuticals, in electroplating, and as a polymerization inhibitor. Other applications include uses as a corrosion inhibitor. It was reported noneffective as a skin depigmentator.
Production Methods
Diethylamine is produced using the three methods also used for the manufacture of ethylamine with very slight modification.
Diethylamine Synthesis
1. N,N-Diethyl-3-methylbenzamide + Ethylene Glycol + NaOH + Heat
2. Ethylchloride + Ammonia (forms diethylamine HCL)
3. Ethanol + Ammonia + Sulfuric Acid
The most widely used method is the passing of ammonia and ethanol over a catalyst such as alumina or silica (Schweizer et al 1978). Diethylamine can be separated from the mixture by selective distillations and extractions.
Definition
ChEBI: Diethylamine is a secondary aliphatic amine where both N-substituents are ethyl. It is a conjugate base of a diethylammonium.
General Description
A clear colorless liquid with an ammonia-like odor. Density 5.9 lb / gal. Flash point -15°F. A respiratory irritant. Corrosive to the eyes and skin. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion.
Air & Water Reactions
Highly flammable. Soluble in water. Sensitive to heat. May be sensitive to prolonged exposure to air.
Reactivity Profile
It is incompatible with several chemical substances such as strong oxidisers, acids, cellulose nitrate, some metals, and dicyanofuroxan. N-nitrosamines, many of which are known to be potent carcinogens, may be formed when diethylamine comes in contact with nitrous acid, nitrates, or atmospheres with high nitrous oxide concentrations.
Health Hazard
Diethylamine can be harmful if it is inhaled, swallowed, or in contact with skin. Vapors can irritate the eyes and cause irritation of the respiratory tract, leading to coughing and chest pain. Liquid diethylamine can cause severe burns to the eyes and skin. Vision became misty and halos appeared several hours after workmen were exposed to the vapors of amines such as diethylamine (Grant 1986). The edema of the corneal epithelium, which is principally responsible for the disturbances in vision, clears after one or more days, depending on the severity of exposure. Photophobia and discomfort from roughness of the corneal surface also can occur after greater exposure to the amine.
Flammability and Explosibility
Highly flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No hazardous reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Industrial uses
Diethylamine, like many of the other short chain aliphatic amines, has achieved widespread industrial use as an intermediate in the manufacture of a number of commercial products. Among these are included insecticides, pharmaceuticals, textile finishing agents, and corrosion inhibitors (Hawley 1981; Schweizer et al 1978). It is used as a polymerization inhibitor and/or catalyst in the polymer industry and in the manufacture of surfactants and rubber processing accelerators. This amine also is useful as a depilatory agent for animal skins, as a selective solvent for the removal of impurities from oils, fats, and waxes, and as a flotation agent in the petroleum industry (NIOSH/OSHA 1981; HSDB 1989).
Toxicology
Exposure to low vapor concentrations are reported to transiently impair vision in humans (reduction of sensitivity to light): no-effect threshold 0.022 – 0.028 mL/m3 ( 0.08mg/m3 ). The odor threshold in air is ca. 0.02 – 0.3 mL/m3 ( 0.9 mg/m3 ). Transient physiological effects in rats after 90-d exposure to vapors were reported to already be visible at 0.37 – 4.19 mg/m3 with no effects at 0.05 mg/m3 , but with morphological, apparently reversible changes in the lung and cerebral neurons at the highest concentration. On the other hand, 50 mL/m3 (150 mg/m3 ) was the lowest concentration to show mild, but signifi- cant effects in the lung and liver of rabbits on prolonged inhalation. There were no visible signs of toxicity following exposure of rats to 25 mL/m3 (75 mg/m3 ) for 120 d, but a moderate, bronchiolar hyperplasia of lymphoid cells. It was assumed that diethylamine may exert a certain neurotoxic effect, which, however, was not confirmed by others.
Safety Profile
Moderately toxic by ingestion, inhalation, and skin contact. A skin and severe eye irritant. Exposure to strong vapor can cause severe cough and chest pains. Contact with liquid can damage eyes, possibly permanently; contact with skin causes necrosis and vesciculation. A very dangerous fire hazard when exposed to heat, flame, or oxidizers. To fight fire, use alcohol foam, CO2, dry chemical. Explodes on contact with dicyanofurazan. Violent reaction with sulfuric acid. Ignites on contact with cellulose nitrate of sufficiently high surface area. When heated to decomposition it emits toxic fumes of NOx. See also MINES.
Potential Exposure
Tumorigen,Mutagen. Primary Irritant. Diethylamine (DEA) is used in themanufacture of the following chemicals: diethyldithiocarbamate and thiurams (rubber processing accelerators);diethylaminoethanol (medicinal intermediate); diethylaminopropylamine (epoxy curing agent); N,N-diethyl-m-toluamide and other pesticides; and 2-diethylaminoethylmethacrylate. Itis used in the manufacture of several drugs.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
No evidence of mutagenicity was seen
in Ames bacterial assays.8 Diethylamine has
an ammonia-like odor that is detectable at
0.13ppm.
The 2003 ACGIH threshold limit valuetime-
weighted average (TLV-TWA) for
diethylamine is 5ppm (15mg/m3) with
a short-term excursion limit of 15ppm
(45mg/m3) and an A4-not classifiable as a
human carcinogen designation; there is a notation
for skin absorption.
Environmental Fate
Photolytic. Low et al. (1991) reported that the photooxidation of aqueous secondary amine
solutions by UV light in the presence of titanium dioxide resulted in the formation of ammonium
and nitrate ions.
Chemical/Physical. Diethylamine reacted with NOx in the dark forming diethylnitrosamine. In
an outdoor chamber, photooxidation by natural sunlight yielded the following products:
diethylnitramine, diethylformamide, diethylacetamide, ethylacetamide, ozone, acetaldehyde, and
peroxyacetyl nitrate (Pitts et al., 1978).
Reacts with mineral acids forming water-soluble salts (Morrison and Boyd, 1971).
Metabolism
Little information is available regarding the metabolism of diethylamine. The amine can be readily absorbed from the respiratory and gastrointestinal tract. It has been reported that following oral administration of diethylamine hydrochloride to humans, much of the amine was recovered in the urine (Beard and Noe 1978). This suggests that it is not readily metabolized and, therefore, may not be a substrate for monoamine oxidase. When administered intraperitoneally to rats, it was moderately inhibitory with respect to liver monoamine oxidase (Valiev 1974). Diethylamine may serve as a precursor for the formation of the reportedly carcinogenic N-nitrosoamines and, indeed, when a diethylamine containing liquid was examined for nitrosation reactions under simulated conditions of the human stomach, N-nitrosodiethylamine was formed (Ziebarth 1985).
storage
Diethylamine should be protected from physical damage. It should be kept stored in a cool, dry, well-ventilated location, away from incompatible chemical substances and away from fi re hazard and smoking areas. The containers should be bonded and grounded for transfer to avoid static sparks. Storage and use areas should be no smoking areas.
Shipping
This compound requires a shipping label of“FLAMMABLE LIQUID.” It falls in Hazard Class 3 andPacking Group II.
Purification Methods
Dry diethylamine with LiAlH4 or KOH pellets. Reflux with, and distil it from, BaO or KOH. Convert it to the p-toluenesulfonamide and crystallise to constant melting point from dry pet ether (b 90-120o), then hydrolyse with HCl, excess NaOH is added, and the amine is passed through a column of activated alumina. Redistil the amine and dry it with activated alumina before use [Swift J Am Chem Soc 64 115 1942]. [Beilstein 4 III 313.] § A polystyrene diethylaminomethyl supported version is commercially available.
Incompatibilities
Forms explosive mixture with air. Mayaccumulate static electrical charges, and may cause ignitionof its vapors. Violent reaction with strong oxidizers, strongacids, cellulose nitrate. Incompatible with organic anhydrides, isocyanates, vinyl acetate, acrylates, substitutedallyls, alkylene oxides, epichlorohydrin, ketones, aldehydes,alcohols, glycols, mercury, phenols, cresols, caprolactumsolution. Attacks aluminum, copper, lead, tin, zinc, andalloys.
Precautions
Occupational workers and users should be very careful during the use and chemical management of diethylamine. Workers should wear impervious protective clothing, including boots, gloves, a laboratory coat, apron or coveralls, as appropriate, to prevent skin contact. The chemical is very hazardous, corrosive, and harmful, and is a very flammable liquid and vapor. Exposures to vapor may cause fl ash fi re. It causes burns and adverse effects to the cardiovascular system. Workers should use chemical safety goggles and a full-face shield to avoid splashing of the chemical substance. An eye-wash fountain and quickdrench facilities in the work area should be maintained by the chemical management unit.
Diethylamine Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 | sales@tjzxchem.com | China | 559 | 58 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
View Lastest Price from Diethylamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Diethylamine
109-89-7
|
US $20.00 / kg | 1kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-24 | Diethylamine
109-89-7
|
US $13.00 / kg | 10kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-01-08 | Diethylamine
109-89-7
|
US $2.00 / mL | 1mL | 99% | 50000tons | Wuhan Boyuan Import & Export Co., LTD |
-

- Diethylamine
109-89-7
- US $20.00 / kg
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Diethylamine
109-89-7
- US $13.00 / kg
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Diethylamine
109-89-7
- US $2.00 / mL
- 99%
- Wuhan Boyuan Import & Export Co., LTD
109-89-7(Diethylamine)Related Search:
1of4





