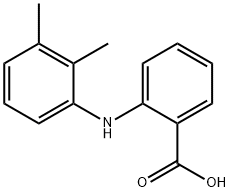
Mefenamic acid
- CAS No.
- 61-68-7
- Chemical Name:
- Mefenamic acid
- Synonyms
- Mefenamic;Mefanamic acid;MEFENAMATE;Ponstel;Ponstan;ac.mefenamico;Mephenamic acid;Mefenamic Acid (200 mg);in-M;HL 1
- CBNumber:
- CB5472051
- Molecular Formula:
- C15H15NO2
- Molecular Weight:
- 241.29
- MDL Number:
- MFCD00051721
- MOL File:
- 61-68-7.mol
- MSDS File:
- SDS
| Melting point | 230 °C |
|---|---|
| Boiling point | 384.06°C (rough estimate) |
| Density | 1.0944 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, slightly soluble in ethanol (96 per cent) and in methylene chloride. It dissolves in dilute solutions of alkali hydroxides |
| pka | 4.2(at 25℃) |
| color | White to Pale Yellow |
| Water Solubility | It is soluble in acetone, chloroform, dichloromethane, methanol. Insoluble in water. |
| Merck | 14,5798 |
| InChIKey | HYYBABOKPJLUIN-UHFFFAOYSA-N |
| LogP | 5.120 |
| CAS DataBase Reference | 61-68-7(CAS DataBase Reference) |
| FDA UNII | 367589PJ2C |
| ATC code | M01AG01 |
| NIST Chemistry Reference | Mefenamic acid(61-68-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-40-20/21/22 | |||||||||
| Safety Statements | 22-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CB4550000 | |||||||||
| HS Code | 28142000 | |||||||||
| Toxicity | LD50 orally in mice, rats: 630, 790 mg/kg (Jahn, Adrian) | |||||||||
| NFPA 704 |
|
Mefenamic acid price More Price(39)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 92574 | Mefenamic acid analytical standard | 61-68-7 | 250mg | $85.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1379605 | Mefenamic acid United States Pharmacopeia (USP) Reference Standard | 61-68-7 | 200mg | $436 | 2024-03-01 | Buy |
| TCI Chemical | M1782 | Mefenamic Acid >98.0%(T) | 61-68-7 | 25g | $30 | 2024-03-01 | Buy |
| TCI Chemical | M1782 | Mefenamic Acid >98.0%(T) | 61-68-7 | 100g | $76 | 2024-03-01 | Buy |
| Alfa Aesar | J62705 | Mefenamic acid, 98% | 61-68-7 | 25g | $34.65 | 2024-03-01 | Buy |
Mefenamic acid Chemical Properties,Uses,Production
Description
Mefenamic acid is a kind of nonsteroidal anti-inflammatory (NSAID) drug belonging to the anthranilic acid derivatives class. It is mainly used for the short-term treatment of mild to moderate pain from various conditions. It is also used for reducing the pain and blood loss from menstrual condition as well as prevention of migraines. Moreover, it may also be used for treating gout attacks. Its mechanism is through inhibiting both the isoforms of COX and preventing the formation of prostaglandins. It is manufactured from 2-chlorobenzoic acid and 2,3-dimethylaniline.
References
http://www.webmd.com/drugs/2/drug-11586/mefenamic-acid-oral/details
https://en.wikipedia.org/wiki/Mefenamic_acid
Chemical Properties
white or light yellow crystalline powder, odorless, insoluble in water, slightly soluble in ethanol, chloroform, slightly soluble in ether. Melting point 230-231°C, mefenamic acid is an anti-inflammatory analgesic with antipyretic, analgesic and anti-inflammatory effects.
Originator
Ponstan,Parke Davis,UK,1963
Uses
Mefenamic acid is used for the same indications as flufenamic acid. Synonyms for this drug are parkemed, ponstan, ponstel, and others.
Uses
For the treatment of rheumatoid arthritis, osteoarthritis, dysmenorrhea, and mild to moderate pain, inflammation, and fever.
Indications
Mefenamic acid (Ponstel) is indicated only for analgesia and primary dysmenorrhea when therapy will not exceed 1 week.
Definition
ChEBI: An aminobenzoic acid that is anthranilic acid in which one of the hydrogens attached to the nitrogen is replaced by a 2,3-dimethylphenyl group. Although classed as a non-steroidal anti-inflammatory drug, its anti-inflammatory properties are considered to b minor. It is used to relieve mild to moderate pain, including headaches, dental pain, osteoarthritis and rheumatoid arthritis.
Manufacturing Process
A mixture of 800 g of potassium o-bromo-benzoate, 1,500 ml of bis-(2- methoxyethyl)ether, 355 g of N-ethyl-morpholine, 375 g of 2,3- dimethylaniline, and 30 g of cupric acetate is heated gradually with stirring to 140°C over a period of 90 minutes. The hot reaction mixture is then acidified with 260 mi of concentrated hydrochloric acid and the acidified mixture divided into 2 equal portions. One liter of water is added to each portion and the mixtures allowed to cool. The N-(2,3-dimethylphenyl)anthranilic acid which separates upon cooling is collected by filtration and recrystallized from bis(2-methoxyethyl)ether; MP 229° to 230°C (corr.).
brand name
Ponstel (Sciele, Parke Davis, USA), Lysalgo (SIT, Italy), Opustan (Opus Pharm, UK), Parkemed (Parke Davis, Germany), Ponstan (Werner-Lambert, Switzerland), Pontal (Sankyo, Japan).
Therapeutic Function
Analgesic
Synthesis Reference(s)
The Journal of Organic Chemistry, 45, p. 2127, 1980 DOI: 10.1021/jo01299a020
General Description
Mefenamic acid (Ponstel, Ponstan) is one of the oldestNSAIDs, introduced into the market in 1967 for mild tomoderate pain and for primary dysmenorrhea. It is rapidly absorbed with peak plasma levels occurring 2 to 4 hoursafter oral administration. It undergoes hepatic benzylic hydroxylationof its 3'methyl group regioselectively into twoinactive metabolites, 3'-hydroxymethylmefenamic acid andthe 3'carboxylate metabolite (via further oxidation of thebenzylic alcohol group). The parent drugs and these metabolitesare conjugated with glucuronic acid and excreted primarilyin the urine. Thus, although patients with knownliver deficiency may be given lower doses, it is contraindicatedin patients with preexisting renal dysfunction.
Common side effects associated with its use include diarrhea,drowsiness, and headache. The possibility of blood disordershas also prompted limitation of its administration to 7days. It is not recommended for children or during pregnancy.
Biochem/physiol Actions
Mefenamic acid is an analgesic and anti-inflammatory drug. It acts as a cyclooxygenase (COX) enzyme inhibitor. It is hepatoxic and implicated in liver injury. Contrarily, mefenamic acid elicits neuroprotection in in vivo ischemic stroke models by inhibiting cell toxicity induced by glutamate. Mefenamic due its inhibitory effect on prostaglandin synthesis can be used in reducing edema and ache.
Mechanism of action
Mefenamic acid inhibits both COX isoforms with some preference for COX-2 and modifies ion channels.
Clinical Use
Mefenamic acid is synthesized from o-chlorobenzoic acid and 2,3-dimethylaniline under catalytic conditions. Mefenamic acid is the only fenamic acid derivative that produces analgesia centrally and peripherally. Mefenamic acid is indicated for the short-term relief of moderate pain and for primary dysmenorrhea.
Safety
Mefenamic acid has mild anti-inflammatory properties and is used primarily as a short-term analgesic. Gastrointestinal disturbances, including possibly allergic diarrhea and potential renal toxicity, limit its use.
Synthesis
Mefenamic acid, N-(2,3-xylyl)anthranylic acid (3.2.19), is synthesized
in basically the same manner, by the reaction of the potassium salt of 2-bromobenzoic acid
with 2,3-dimethylaniline in the presence of copper (II) acetate [80,81].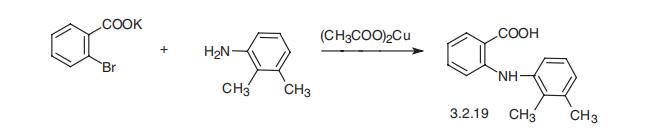
Synthesis 2: mefenamic acid is prepared via
the Jourdan – Ullmann – Goldberg synthesis utilizing either anthranilic acid and 3-bromo-1,2-
dimethylbenzene or 2,3-dimethylaniline and an
o-halobenzoic acid in the presence of a copper
catalyst and a proton acceptor.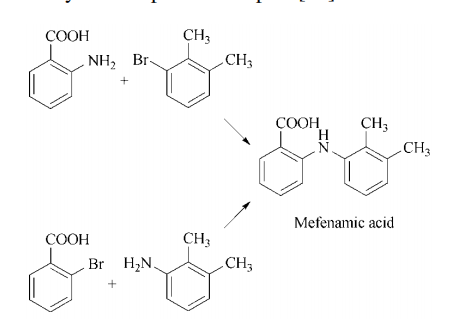
Metabolism
Mefenamic acid is absorbed rapidly following oral administration, with peak plasma levels being attained within 2 to 4 hours. It is highly bound to plasma proteins (78.5%) and has a plasma half-life of 2 to 4 hours. Metabolism occurs through regioselective oxidation of the 3′-methyl group and glucuronidation of mefenamic acid and its metabolites. Urinary excretion accounts for approximately 50 to 55% of an administered dose, with unchanged drug accounting for 6%, the 3′-hydroxymethyl metabolite (primarily as the glucuronide) accounting for 25%, and the remaining 20% as the dicarboxylic acid (of which 30% is the glucuronide conjugate). These metabolites are essentially inactive.
Mefenamic acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 | Sara@xmwonderfulbio.com | China | 305 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Sigma Audley | +86-18336680971 +86-18126314766 | nova@sh-teruiop.com | China | 525 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 893 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
View Lastest Price from Mefenamic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Mefenamic Acid
61-68-7
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-05 | Mefenamic Acid
61-68-7
|
US $0.00 / kg | 1kg | 99% | 1000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-03-19 | Mefenamic acid
61-68-7
|
US $45.00-35.00 / kg | 1kg | 99.8% | 200tons/year | Sigma Audley |
-

- Mefenamic Acid
61-68-7
- US $0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- Mefenamic Acid
61-68-7
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Mefenamic acid
61-68-7
- US $45.00-35.00 / kg
- 99.8%
- Sigma Audley
61-68-7(Mefenamic acid )Related Search:
1of4