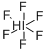IODINE HEPTAFLUORIDE
- CAS No.
- 16921-96-3
- Chemical Name:
- IODINE HEPTAFLUORIDE
- Synonyms
- IF7;Einecs 240-981-4;iodinefluoride(if7);IODINE HEPTAFLUORIDE;Seven fluoride iodine;heptafluoro-λ7-iodane;Iodine fluoride (IF7);IODINE HEPTAFLUOROIDE;Iodine heptrafluoride;Iodine(VII)heptafluoride
- CBNumber:
- CB5491090
- Molecular Formula:
- F6HI
Lewis structure

- Molecular Weight:
- 241.9
- MDL Number:
- MFCD25978139
- MOL File:
- 16921-96-3.mol
| Melting point | 6.45° |
|---|---|
| Boiling point | 4,7°C |
| Density | 2,6 g/cm3 |
| solubility | soluble in H2O |
| form | gas |
| color | colorless |
| Water Solubility | soluble H2O with some decomposition [MER06] |
| FDA UNII | M080M03ILM |
| EPA Substance Registry System | Iodine fluoride (IF7) (16921-96-3) |
SAFETY
Risk and Safety Statements
| Risk Statements | 8-14-34 |
|---|---|
| Safety Statements | 3/7-9-17-36/37/39-45 |
| RIDADR | 3157 |
IODINE HEPTAFLUORIDE Chemical Properties,Uses,Production
Chemical Properties
colorless liquid; enthalpy of vaporization 24.7kJ/mol; specific conductivity 10?9ohm· cm [KIR78] [MER06]
Uses
Iodine heptafluoride is used as a fluorinatingagent.
Uses
Iodine heptafluoride may be prepared by the reaction of fluorine withpotassium iodide:
5I2 + 4HCl + 3Cl2 → 10ICl + 2H2
Dried KI should be used to minimize the formation of IOF5.
Also, IF7 can be prepared by passing fluorine gas through liquid iodinepentafluoride at high temperature (90°C) and then heating the vapors to 270°C to complete the reaction:
2I2 + HIO3 + 2HCl → 2ICl + 3HIO
Health Hazard
Iodine heptafluoride is highly irritating to theskin and mucous membranes. Toxicity dataon this compound are not available.
Fire Hazard
Nonflammable gas. It reacts violently with ammonium halides (chloride, bromide, or iodide) and with organic matter (Mellor 1946, Suppl. 1956). It burns with carbon monoxide.
IODINE HEPTAFLUORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 4006608290; 18621169109 | market03@meryer.com | China | 40241 | 62 |
| HUNAN CHEMFISH SCIENTIFIC CO.,LTD | +86-0731-85567275 13021512891 | sales02@chemfish.com | China | 6582 | 58 |
| Isotope (Xiamen) Industry and Trade Co., Ltd | 0510-051082803581 15903302207 | zhongxin326@sohu.com | China | 229 | 58 |
| DWS Specialty Gas Co., Ltd | 159-0619-7626 13194677939 | yanning@abamtc.com | China | 454 | 58 |
| Hubei Enxing Biotechnology Co., Ltd | 16621771607 | exbio_tech@163.com | China | 8364 | 58 |