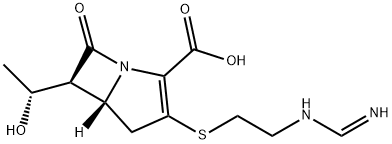
Imipenem
- CAS No.
- 64221-86-9
- Chemical Name:
- Imipenem
- Synonyms
- imipenam;Imipenen;Imipenem CRS;(5R)-6β-[(R)-1-Hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;(5R,6S)-3-({2-[(E)-(aMinoMethylidene)aMino]ethyl}sulfanyl)-6-[(1R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;mk-787;Imipenem;LMipeneM;imipemide;EOS-62374
- CBNumber:
- CB5695768
- Molecular Formula:
- C12H17N3O4S
- Molecular Weight:
- 299.35
- MDL Number:
- MFCD00864955
- MOL File:
- 64221-86-9.mol
| Melting point | 106-111 C |
|---|---|
| Boiling point | 530.2±60.0 °C(Predicted) |
| Density | 1.62±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | Sparingly soluble in water, slightly soluble in methanol. |
| pka | 4.29±0.40(Predicted) |
| CAS DataBase Reference | 64221-86-9(CAS DataBase Reference) |
| FDA UNII | Q20IM7HE75 |
| EPA Substance Registry System | Imipenem (64221-86-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P280-P261 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-27-36/37/39 |
| HS Code | 29419000 |
Imipenem price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Alfa Aesar | J66272 | Imipenem mixturewithcilastatin | 64221-86-9 | 25mg | $698 | 2021-12-16 | Buy |
| SynQuest Laboratories | 8H66-1-21 | Imipenem | 64221-86-9 | 1g | $412 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 18663 | Imipenem | 64221-86-9 | 5mg | $950 | 2021-12-16 | Buy |
| AHH | API-1080 | Imipenem 98% | 64221-86-9 | 1g | $510 | 2021-12-16 | Buy |
| Crysdot | CD11081600 | Imipenem 95+% | 64221-86-9 | 1g | $585 | 2021-12-16 | Buy |
Imipenem Chemical Properties,Uses,Production
Description
Imipenem is a chemically stable thienamycin derivative with an antibacterial activity that is broader in spectrum and of greater potency than most of the third generation cephalosporins. Combination with cilastatin, an inhibitor of renal brush-border dehydropeptidase-I, increases both urinary and plasma levels of imipenem.
Chemical Properties
White Crystals
Originator
Merck (USA)
Uses
Carbapenem antibacterial.
Definition
ChEBI: Imipenem is a broad-spectrum, intravenous beta-lactam antibiotic of the carbapenem subgroup. It has a role as an antibacterial drug. It is a beta-lactam antibiotic allergen and a member of carbapenems. It is a tautomer of an imipenem zwitterion.
Manufacturing Process
Preparation of N-formimidoyl thienamycin:
Thienamycin (517 mg) is dissolved in pH 7 0.1 N phosphate buffer (25 ml)
and cooled in an ice bath with magnetic stirring. The solution is adjusted to
pH 8.5 using 2.5 N sodium hydroxide solution dispensed from an automatic
burette. While maintaining a pH of 8.5, methyl formimidate hydrochloride
(711 mg) is added portionwise over 2-3 minutes. After an additional 10 min,
the pH of the solution is brought to 7.0 using 2.5 N hydrochloric acid. The
solution is chromatographed on a column of XAD-2 resin (150 ml) which is
eluted with water. The N-formimidoyl thienamycin derivative (imipenem)
elutes in 1.5-2.0 column volumes (200-300 ml) and is lyophilized to a white
solid (217 mg). UV (pH 7 0.1 N phosphate buffer); λmax297 nm (8,590); ir
(Nujol mull) 1767 Cm-1(β-lactam).
Another method preparation of imipenem:
6-(1)-Hydroxyethyl-1-azabicyclo(3.2.0)heptane-3,7-dione-2-carboxylate is
converted to the diphenoxyphosphate enol ester and this in turn reacted with
N,S-bistrimethylsilyl-N-formimidoylcysteamine (use of the bistrimethylsilylated
reagent is necessary in order to avoid side reactions caused by cyclization
reactions). As a result the (PhO)2OPO-groups are converted to Me3SiN =
CHNH-groups. Removal of the protecting groups complete the synthesis of 1-
azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 6-(1-hydroxyethyl)-3-((2-
((iminomethyl)amino)ethyl)thio)-7-oxo-, (5R-(5-alpha,6-alpha(R*)))-.
brand name
ZIENAM
Therapeutic Function
Antibiotic
Antimicrobial activity
Imipenem shows potent activity against a wide range of Grampositive
and Gram-negative aerobes and anaerobes, including
many resistant to other agents.Concentrations
(mg/L) inhibiting 50% of strains of other organisms are:
Listeria monocytogenes, 0.03; Legionella pneumophila, 0.03;
Enterococcus faecium, 4; Yersinia spp., 0.06. Mycobacterium fortuitum
is inhibited by 6.25 mg/L. Imipenem is active against
many Pseudomonas species, but not Sten. maltophilia. It is
active against most anaerobes, with the exception of Cl. perfringens,
which is only moderately susceptible. It is bactericidal
at 2–4 times the MIC for most species, but some strains
of Staph. aureus exhibit ‘tolerance’ . Bactericidal
synergy with aminoglycosides, glycopeptides, fosfomycin and
rifampicin (rifampin) has been observed against many strains
of Staph. aureus and enterococci.
Antibacterial activity is unaffected by the presence of cilastatin,
which is itself devoid of antimicrobial activity.
Imipenem is stable to hydrolysis by most serine β-lactamases,
with the exception of the group 2f carbapenem-hydrolyzingenzymes hydrolyzingenzymes
. Strains of B. fragilis, Aeromonas spp.
and Sten. maltophilia can produce metallo-β-lactamases that
hydrolyze the drug rapidly. These strains, in addition to occasional
strains of enterobacteria, Acinetobacter baumannii and
Ps. aeruginosa, show variable resistance to imipenem depending
upon the level of carbapenem-hydrolyzing enzymes and
the presence or absence of imipenem-specific porins. Efflux
pumps also exist that may extrude imipenem from Gramnegative
bacteria.
Acquired resistance
Some strains of Citrobacter, Enterobacter, Proteus vulgaris,
Providencia, Ps. aeruginosa and Serratia spp. may be resistant
to imipenem and other β-lactam agents, often because
of the selection of stably derepressed mutants expressing
high levels of group 1 β-lactamases coupled with decreased
intracellular drug levels due to porin mutations or increased
efflux.
Induction of class 1 β-lactamases by imipenem in strains
of Aeromonas, Pseudomonas and Serratia spp. is responsible
for antagonism of β-lactamase-labile β-lactam agents in vitro.
Imipenem resistance in Ps. aeruginosa can occur following
selection of mutants that hyperproduce the group 1 cephalosporinase
and which are also deficient in an outer membrane
protein (OprD or D2) which specifically transports
imipenem, but not cephalosporins or monobactams.
General Description
Thienamycin was found in the culture broth of Streptomyces cattleya by Merck Sharp & Dohme in 1976, as a very unstable substance. It has a unique carbapenem structure, like that of the olivanic acids found in S. olivaceus by Beecham Research Laboratories in 1979. Thienamycin shows excellent activity against a variety of pathogenic bacteria, including Pseudomonas aeruginosa. Its chemical stability has been improved by derivatization with the formimidoyl group, and its biological stability has been improved by combining it with cilastatin, an inhibitor of kidney dihydropeptidase. The combination drug imipenem – cilastatin is now under study to evaluate its clinical efficacy and safety.
Clinical Use
Lower respiratory tract infections
Urinary tract infections (complicated and uncomplicated)
Intra-abdominal infections
Gynecological infections
Bacterial septicemia
Bone and joint infections
Skin and skin structure infections
Endocarditis
Polymicrobial infections
Side effects
CNS effects such as confusional states and seizures have been
reported, especially when recommended doses were exceeded,
and in patients with renal failure or creatinine clearances of
≤20 mL/min/1.73 m2.
Other reactions include phlebitis/thrombophlebitis (3.1%),
nausea (2.0%), diarrhea (1.8%) and vomiting (1.5%).
Increased hepatic enzymes may be seen in adults and children.
Superinfection with Aspergillus, Candida and resistant
Pseudomonas spp. have been described and pseudomembranous
colitis has been reported.
Patients with a history of hypersensitivity reactions to penicillins,
cephalosporins or other β-lactam antibiotics should be
treated cautiously with carbapenems.
Imipenem Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3734 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
Related articles
- Imipenem: Antimicrobial Activity, Susceptibility, Administration and Dosage, Toxicity, Clinical Uses etc.
- In the 1970s, Beecham Research Laboratories identified a carbapenem group, called olivanic acids, which were beta-lactamase in....
- Mar 25,2022
View Lastest Price from Imipenem manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-20 | imipenem
64221-86-9
|
US $10.00 / g | 1g | 98% | 50kg | Wuhan Topule Biopharmaceutical Co., Ltd | |
 |
2023-08-30 | Imipenem
64221-86-9
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-02-13 | Imipenem
64221-86-9
|
US $100.00 / kg | 1kg | 99% | 50MT | Hebei baicao biology science and technology co., ltd |
64221-86-9(Imipenem)Related Search:
1of4