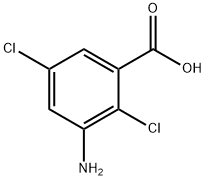
Chloramben
- CAS No.
- 133-90-4
- Chemical Name:
- Chloramben
- Synonyms
- AMBEN;AMIBEN;Amibin;Amoben;Naptol;Ambiben;Vegaben;Vegiben;Acpm-629;amibends
- CBNumber:
- CB5702082
- Molecular Formula:
- C7H5Cl2NO2
- Molecular Weight:
- 206.03
- MDL Number:
- MFCD00065093
- MOL File:
- 133-90-4.mol
- MSDS File:
- SDS
| Melting point | 200 °C |
|---|---|
| Boiling point | 312 °C |
| Density | 1.4062 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| storage temp. | 0-6°C |
| solubility | Acetonitrile (Slightly), DMSO (Sparingly), Metahnol (Slightly) |
| pka | 2.75±0.25(Predicted) |
| form | Powder |
| color | Pale Beige |
| Water Solubility | 700 mg/L (25 ºC) |
| Merck | 13,2082 |
| BRN | 2365906 |
| Exposure limits | An experimental carcinogen. |
| CAS DataBase Reference | 133-90-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | EWG424FFB5 |
| NIST Chemistry Reference | 3-Amino-2,5-dichlorobenzoic acid(133-90-4) |
| Pesticides Freedom of Information Act (FOIA) | Chloramben |
| EPA Substance Registry System | Chloramben (133-90-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H319-H335-H350 | |||||||||
| Precautionary statements | P201-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | T,Xn | |||||||||
| Risk Statements | 45-36/37/38-40 | |||||||||
| Safety Statements | 53-22-26-36/37/39-45-36 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DG1925000 | |||||||||
| HS Code | 29224999 | |||||||||
| Toxicity | Nontoxic to fish (Hartley and Kidd, 1987); acute oral LD50 for rats 5,620 mg/kg (Hartley and Kidd, 1987), 3,500 mg/kg (RTECS, 1985). | |||||||||
| NFPA 704 |
|
Chloramben price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 152668 | 3-Amino-2,5-dichlorobenzoic acid 95% | 133-90-4 | 5g | $196.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 33392 | Chloramben PESTANAL?, analytical standard | 133-90-4 | 100mg | $74.2 | 2022-05-15 | Buy |
| TCI Chemical | A1537 | 3-Amino-2,5-dichlorobenzoic Acid | 133-90-4 | 5G | $159 | 2024-03-01 | Buy |
| TRC | A605273 | 3-Amino-2,5-dichlorobenzoic acid | 133-90-4 | 5g | $185 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FA70665 | 3-Amino-2,5-dichlorobenzoic acid | 133-90-4 | 2g | $50 | 2021-12-16 | Buy |
Chloramben Chemical Properties,Uses,Production
Description
Chloramben is a colorless, odorless, crystallinesolid. Molecular weight=206.03; Freezing/Meltingpoint=200-201℃; Vapor pressure=6.8 3 1023 mmHg at100℃. Hazard Identification (based on NFPA 704 MRating System): Health 3, Flammability 0, Reactivity 0.Soluble in water; solubility=690 ppm at 25℃.
Chemical Properties
Off-white to beige powder
Chemical Properties
Chloramben is a colorless, odorless, crystalline solid.
Uses
Chloramben may be used as an analytical reference standard for the determination of the analyte in water samples using liquid chromatography with electrochemical detection (LC-EC).
Uses
Preemergence or preplant herbicide used in many vegetable and field crops to control annual broad-leaved weeds and grasses. Also for postemergent control of common ragweed, redroot pigweed, smartweed and velvet-leaf.
Uses
Herbicide or plant growth regulator.
Definition
ChEBI: Chloramben is a chlorobenzoic acid.
General Description
Purplish white powder or light purple solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Chloramben reacts with sodium hypochlorite solutions .
Health Hazard
ACUTE/CHRONIC HAZARDS: Chloramben emits toxic fumes when heated to decomposition.
Fire Hazard
Flash point data for Chloramben is not available, but Chloramben is probably combustible.
Agricultural Uses
Herbicide: A herbicide for grasses, and broadleaf weeds. Mostly used on soybeans, and also on corn, beans, asparagus, pumpkins, peanuts, sunflowers, peppers, cotton, sweet potatoes, squash, melons, hardwood trees, and some conifers. Not approved for use in EU countries. Not registered for use in the U.S. There are 51 global suppliers
Trade name
ACP-M-728®; AMBEN®; AMBIBEN®; AMIBEN®[C]; AMIBIN®; AMOBEN®; ORNAMENTAL WEEDER®[C]; VEGABEN®; VEGIBEN®[C]; WEEDONE® GARDEN WEEDER
Potential Exposure
A General Use Pesticide (GUP) that is no longer produced or sold in the United States. It is used as an herbicide for grasses, broadleaf weeds, soybeans, beans, and some vegetables. Workers involved in the manufacture, formulation, or application of this reemergence herbicide.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Environmental Fate
Soil. In soils, chloramben was degraded by microorganisms but no products were
identified (Humburg et al., 1991). The main degradative pathway of chloramben in soil is
decarboxylation and subsequent mineralization to carbon dioxide. The calculated halflives in Ella loamy sand, Kewaunee clay and Poygan silty clay were 120–201, 182–286
and 176–314 days, respectively (Wildung et al., 1968). Persistence in soil is 6–8 weeks
(Hartley and Kidd, 1987).
Groundwater. According to the U.S. EPA (1986) and Ashton and Monaco (1991),
chloramben has a high potential to leach to groundwater, especially in sandy soils during
heavy rains.
Plant. Degrades in plants to N-glucoside, glucose ester, conjugates and insoluble
residues (Ashton and Monaco, 1991).
Photolytic. Plimmer and Hummer (1969) studied the irradiation of chloramben in water
(2–4 mg/L) under a 450-W mercury vapor lamp (λ >2,800 ?) for periods of 2 to 20 hours.
Chloride ion was released and a complex mixture of colored products was observed. It
was postulated that amino free radicals reacted with each other via polymerization and
oxidation processes. The experiment was repeated except the solution contained sodium
bisulfite as an inhibitor under a nitrogen atmosphere. Oxidation did not occur and loss of
the 2-chloro substituent gave 3-amino-5-chlorobenzoic acid (Plimmer and Hummer, 1969).
Chloramben (sodium salt) in aqueous solutions (100 mg/L) was rapidly photodegraded
in outdoor sunlight and under a 360-W mercury arc lamp (Crosby and Leitis, 1969).
In
sunlight, the solution became yellow-brown. Subsequent analysis by gas-liquid chromatography did not resolve any compounds other than chloramben. However, analysis by
TLC indicated at least 12 unidentified products. These products were reportedly formed
via replacement of chlorine by a hydroxy group, reductive dechlorination and abstraction
of hydrogen from the amine group (oxidation). No photodegradation products could be
identified in the solutions irradiated with the mercury arc lamp (Crosby and Leitis, 1969).
Chemical/Physical. Emits toxic fumes of nitrogen oxides and chlorine when heated
to decomposition (Sax and Lewis, 1987).
Forms water-soluble salts with alkalies.
Metabolism
Chloramben is generally stable to hydrolytic degradation; however, it will decompose in sodium hypochlorite solutions. It is also very sensitive to light and under oxidative conditions aqueous solutions will rapidly undergo photolysis. The predominant reaction in oxidative photolysis is unclear but appears to involve the formation of amino radicals. Photolysis also occurs under reductive conditions by dechlorination at the 2 position, i.e., when appropriate reducing agents are present, e.g., sodium bisulfite.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with chloramben you should be trained on its proper handling and storage. Store in a cool, dry place, or a refrigerator, and avoidcontact with strong acids, acid fumes, and light. A regulated, marked area should be established where this chemical is handled, used, or stored in compliance with OSHAStandard 1910.1045.
Shipping
UN2588 Pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Toxicity evaluation
Although limited studies are available, those that have been conducted, for example, on cows and dogs, indicate that the majority of ingested chloramben is quickly excreted through the urine and feces and does not accumulate in tissues. An interesting interaction between caffeine and chloramben suggests that they can form an insoluble complex in the intestinal tract and thus decrease the amount of chloramben absorbed into the blood stream. The acute oral LD50 in rat is >5000 mg/kg.
Incompatibilities
Rapidly decomposed by light. Strong acids and acid fumes
Waste Disposal
Chloramben is stable to heat, oxidation, and hydrolysis in acidic or basic media. The stability is comparable to that of benzoic acid. Wet oxidation or incineration are recommended disposal methods.
Chloramben Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| GLR Innovations | +91 9891111994 | info@glrgroup.in | India | 4542 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
View Lastest Price from Chloramben manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-03-06 | chloramben
133-90-4
|
US $0.00 / KG | 1KG | 99% | 10 ton | Hebei Guanlang Biotechnology Co,.LTD |
-

- chloramben
133-90-4
- US $0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co,.LTD