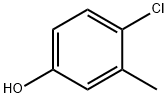
4-Chloro-3-methylphenol
- CAS No.
- 59-50-7
- Chemical Name:
- 4-Chloro-3-methylphenol
- Synonyms
- Chlorocresol;PCMC;4-CMC;CMK;PARA CHLORO META CRESOL;4-CHLORO-3-CRESOL;Parmetol;4-CHLORO-M-CRESOL;CMPK;chlorocresols
- CBNumber:
- CB5703115
- Molecular Formula:
- C7H7ClO
- Molecular Weight:
- 142.58
- MDL Number:
- MFCD00002323
- MOL File:
- 59-50-7.mol
- MSDS File:
- SDS
| Melting point | 63-65 °C (lit.) |
|---|---|
| Boiling point | 235 °C (lit.) |
| Density | 1.370 |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index | 1.5449 (estimate) |
| Flash point | 118 °C |
| storage temp. | Store below +30°C. |
| solubility | methanol: soluble1g/10 mL, clear, colorless |
| form | Tablets |
| pka | pKa 9.55(t = 25) (Uncertain) |
| color | White |
| PH | 6.5 (1g/l, H2O, 20℃) |
| Water Solubility | 4 g/L (20 ºC) |
| Merck | 14,2133 |
| BRN | 1237629 |
| Henry's Law Constant | 2.5(x 10-6 atm?m3/mol)at 20 °C (calculated, Mabey et al., 1982) |
| Stability | Stable. Incompatible with brass, oxidizing agents, copper, copper alloys. |
| InChIKey | CFKMVGJGLGKFKI-UHFFFAOYSA-N |
| LogP | 3.100 |
| Indirect Additives used in Food Contact Substances | 4-CHLORO-3-METHYLPHENOL |
| FDA 21 CFR | 175.105; 176.200 |
| CAS DataBase Reference | 59-50-7(CAS DataBase Reference) |
| EWG's Food Scores | 5 |
| FDA UNII | 36W53O7109 |
| NIST Chemistry Reference | Phenol, 4-chloro-3-methyl-(59-50-7) |
| EPA Substance Registry System | p-Chloro-m-cresol (59-50-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H312-H314-H317-H335-H410 | |||||||||
| Precautionary statements | P260-P273-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N,T,F | |||||||||
| Risk Statements | 21/22-41-43-50-39/23/24/25-23/24/25-11-38 | |||||||||
| Safety Statements | 26-36/37/39-61-45-36/37-16-7-28 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | GO7100000 | |||||||||
| Autoignition Temperature | 590 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29081000 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1830 mg/kg LD50 dermal Rat > 2000 mg/kg | |||||||||
| NFPA 704 |
|
4-Chloro-3-methylphenol price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C55402 | 4-Chloro-3-methylphenol 99% | 59-50-7 | 5g | $43.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02295 | 4-Chloro-3-methylphenol for synthesis | 59-50-7 | 5G | $25 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02295 | 4-Chloro-3-methylphenol for synthesis | 59-50-7 | 250g | $34.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02295 | 4-Chloro-3-methylphenol for synthesis | 59-50-7 | 1kg | $97.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02295 | 4-Chloro-3-methylphenol for synthesis | 59-50-7 | 50kg | $2250 | 2024-03-01 | Buy |
4-Chloro-3-methylphenol Chemical Properties,Uses,Production
Chemical Properties
Colorless or almost colorless, dimorphous crystals or crystalline powder with a characteristic phenolic odor.
Chemical Properties
White (pure) or pink crystalline solid. Crystals turn pink on exposure to air and light. Phenolic odor.
Chemical Properties
white or pink crystals
Physical properties
Colorless, white, or pinkish crystals with a slight phenolic odor. On exposure to air it slowly becomes light brown.
Uses
Disinfectant; pharmaceutic aid (preservative).
Uses
antiinfectant
Uses
P-chloro-m-cresol is used as a preservative in a wide number of topical preparations and is a rare cause of allergic contact dermatitis and CoU, the mechanism of which remains uncertain.
Uses
4-Chloro-3-methylphenol has been used in paint formulations.
Definition
ChEBI: 4-chloro-m-cresol is a hydroxytoluene that is 3-methylphenol which is substituted by a chlorine at position 4. A ryanodine receptor agonist. It has a role as a ryanodine receptor agonist, an antimicrobial agent and a disinfectant. It is a hydroxytoluene and a member of monochlorobenzenes.
Production Methods
Chlorocresol is prepared by the chlorination of m-cresol.
General Description
A pinkish to white crystalline solid with a phenolic odor. Melting point 64-66°C. Shipped as a solid or in a liquid carrier. Soluble in aqueous base. Toxic by ingestion, inhalation or skin absorption. Used as an external germicide. Used as a preservative in paints and inks.
Air & Water Reactions
Hygroscopic. Soluble in aqueous base.
Reactivity Profile
4-Chloro-3-methylphenolS are incompatible with bases, acid chlorides, acid anhydrides, and oxidizing agents. Corrodes steel, brass, copper and copper alloys .
Hazard
Irritant to skin.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Pharmaceutical Applications
Chlorocresol is used as an antimicrobial preservative in cosmetics and pharmaceutical formulations. It is generally used in concentrations up to 0.2% in a variety of preparations except those intended for oral administration or that contact mucous membrane. Chlorocresol is effective against bacteria, spores, molds, and yeasts; it is most active in acidic media. Preservative efficacy may be reduced in the presence of some other excipients, particularly nonionic surfactants.
Contact allergens
Chlorocresol is a biocide used for its disinfectant and preservative properties, in topicals or cutting fluid.
Side effects
Chlorocresol is used as a preservative in a variety of topical preparations, such as corticosteroid creams and moisturizers and in disinfectants and detergents. Three case reports implicate chlorocresol as a cause of Cou; however, whether this is due to an immunological cause is uncertain.
Walker et al. report a patient who experienced localized Cou to a number of topical medicaments and moisturizers within 30 minutes of application. Patch tests of chlorocresol and her own preparations containing chlorocresol applied for just 30 minutes produced marked urticarial responses.
A woman working in an aviary developed eyelid edema and erythema every time she used two specific disinfectants. Open and skin prick testing to 10%, but not 1%, chlorocresol was positive in this case and negative in 10 controls. This patient also experienced eyelid involvement as well as local reactions to the testing, both with superficial necrosis. Freitas et al. acknowledged that it was unusual on both aspects: for such a high concentration to be required to elicit an urticarial reaction and for superficial necrosis to occur.
A case of simultaneous delayed and immediate hypersensitivity has also been reported. Goncalo et al. report a 35-year-old laboratory worker exposed to chlorocresol in both detergents and corticosteroid creams. Patch testing was positive and the patient was diagnosed with allergic contact dermatitis to chlorocresol, which was present in numerous products. Open and skin prick testing to 1% and 5% chlorocresol were positive after 20 minutes. Ten controls were also tested, all were negative to the 1% formulation, although six were positive to 5%,[48] suspicious for a nonimmunological Cou.
Safety Profile
Poison by intravenous, subcutaneous, and intraperitoneal routes. Moderately toxic by ingestion. An allergen. Mutation data reported. Incompatible with sodium hydroxide. When heated to decomposition it emits toxic fumes of Cl and phosgene. See also CRESOL and CHLOROPHENOLS.
Safety
Chlorocresol is used primarily as a preservative in topical
pharmaceutical formulations but has also been used in nebulized
solutions and ophthalmic and parenteral preparations. It should
not, however, be used in formulations for intrathecal, intracisternal,
or peridural injection.
Chlorocresol is metabolized by conjugation with glucuronic acid
and sulfate and is excreted in the urine, mainly as the conjugate,
with little chlorocresol being excreted unchanged.
Although less toxic than phenol, chlorocresol may be irritant to
the skin, eyes, and mucous membranes, and has been reported to
cause some adverse reactions when used as an excipient.
Sensitization reactions may follow the prolonged application of
strong solutions to the skin, although patch tests have shown that
chlorocresol is not a primary irritant at concentrations up to 0.2%.
Chlorocresol is recognized as a rare cause of allergic contact
dermatitis. Cross sensitization with the related preservative
chloroxylenol has also been reported. At concentrations of
0.005% w/v, chlorocresol has been shown to produce a reversible
reduction in the ciliary movement of human nasal epithelial cells in
vitro, and at concentrations of 0.1% chlorocresol produces
irreversible ciliostasis; therefore it should be used with caution in
nasal preparations. However, a clinical study in asthma patients
challenged with chlorocresol or saline concluded that preservative
might be used safely in nebulizer solution.
Chlorocresol is approved as safe for use in cosmetics in Europe at
a maximum concentration of 0.2%, although not in products
intended to come in contact with mucous membranes.
Chlorocresol at a concentration as low as 0.05% produces
ocular irritation in rabbits. Despite such reports, chlorocresol
has been tested in ophthalmic preparations.
When used systemically, notably in a heparin injection preserved
with chlorocresol 0.15%, delayed irritant and hypersensitivity
reactions attributed to chlorocresol have been reported.
LD50 (mouse, IV): 0.07 g/kg
LD50 (mouse, oral): 0.6 g/kg
LD50 (mouse, SC): 0.36 g/kg
LD50 (rabbit, dermal): >5 g/kg
LD50 (rat, dermal): >2 g/kg
LD50 (rat, oral): 1.83 g/kg
LD50 (rat, SC): 0.4 g/kg
Potential Exposure
Chlorinated phenol fungicide, microbiocide, and germicide used to control bacteria, yeasts, and fungi.
Environmental Fate
Biological. When p-chloro-m-cresol was statically incubated in the dark at 25 °C with yeast
extract and settled domestic wastewater inoculum, significant biodegradation with rapid
adaptation was observed. At concentrations of 5 and 10 mg/L, 78 and 76% biodegradation,
respectively, were observed after 7 d (Tabak et al., 1981).
Chemical/Physical. At influent concentrations (pH 3.0) of 1.0, 0.1, 0.01, and 0.001 mg/L, the
GAC adsorption capacities were 122, 63, 32, and 17 mg/g, respectively. At pH 5.5 and pH 9.0 at
influent concentrations of 1.0, 0.1, 0.01, and 0.001 mg/L, the GAC adsorption capacities were 124,
85, 58, and 40 mg/g and 99, 38, 15, and 5.5 mg/g, respectively (Dobbs and Cohen, 1980).
storage
Chlorocresol is stable at room temperature but is volatile in steam. Aqueous solutions may be sterilized by autoclaving. On exposure to air and light, aqueous solutions may become yellow colored. Solutions in oil or glycerin may be sterilized by heating at 1608℃ for 1 hour. The bulk material should be stored in a well-closed container, protected from light, in a cool, dry place.
Shipping
UN2669 Chlorocresols solution, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3437 Chlorocresols solid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise the phenol from pet ether or *C6H6. [Beilstein 6 H 381, 6 I 187, 6 II 355, 6 III 1315, 6 IV 2064.]
Incompatibilities
Chlorocresols react with boranes, alkalies, aliphatic amines, amides, nitric acid, sulfuric acid. Contact with oxidizing agents may cause a fire and explosion hazard. Heat produces phosgene, hydrogen chloride and chlorine gases. Corrosive to aluminum, copper, tin, and other chemically active metals.
Incompatibilities
Chlorocresol can decompose on contact with strong alkalis,
evolving heat and fumes that ignite explosively. It is also
incompatible with oxidizing agents, copper, and with solutions of
calcium chloride, codeine phosphate, diamorphine hydrochloride,
papaveretum, and quinine hydrochloride. Chlorocresol is corrosive
to metals and forms complex compounds with transition metal
ions; discoloration occurs with iron salts. Chlorocresol also exhibits
strong sorption or binding tendencies to organic materials such as
rubber, certain plastics, and nonionic surfactants.
Chlorocresol may be lost from solutions to rubber closures, and
in contact with polyethylene may initially be rapidly removed by
sorption and then by permeation, the uptake being temperature
dependent. Presoaking of components may reduce losses due to
sorption, but not those by permeation. Chlorocresol may also
be taken up by polymethylmethacrylate and by cellulose acetate.
Losses to polypropylene or rigid polyvinyl chloride are usually
small.
At a concentration of 0.1%, chlorocresol may be completely
inactivated in the presence of nonionic surfactants, such as
polysorbate 80. However, other studies have suggested an
enhancement of antimicrobial properties in the presence of
surfactants. Bactericidal activity is also reduced, due to
binding, by cetomacrogol, methylcellulose, pectin, or cellulose
derivatives. In emulsified or solubilized systems, chlorocresol
readily partitions into the oil phase, particularly into vegetable oils,
and higher concentrations will be required for efficient preservation.
Waste Disposal
A good candidate for rotary kiln incineration at a temperature range of 820 to 1600C and residence times of seconds for liquids and gases, and hours for solids.
Regulatory Status
Included in the FDA Inactive Ingredients Database (topical creams
and emulsions). Included in nonparenteral and parenteral medicines
licensed in the UK. Included in the Canadian List of Acceptable
Non-medicinal Ingredients.
In Europe, chlorocresol is approved for use in cosmetics at a
maximum concentration of 0.2%; however, it is prohibited for use
in products intended to come into contact with mucous membranes.
In Japan, use of chlorocresol in cosmetics is restricted to a level of
0.5 g/100 g.
4-Chloro-3-methylphenol Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jiangxi Alpha Hi-tech Pharmaceutical Co., Ltd | 510-85010237 | overseamarketing@alphahi-tech.com | CHINA | 41 | 58 |
| Zhejiang Kinso Pharmaceuticals Co., LTD. | +8619857958369 | sales@kinsopharma.com | China | 27 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9352 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
View Lastest Price from 4-Chloro-3-methylphenol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-06 | 4-Chloro-3-methylphenol
59-50-7
|
US $1.00 / kg | 1kg | 99% | 20tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-08-09 | 4-Chloro-3-methylphenol
59-50-7
|
US $10.00 / kg | 1kg | 99% | 500t/month | Henan Bao Enluo International TradeCo.,LTD | |
 |
2023-07-31 | 4-Chloro-3-methylphenol
59-50-7
|
US $10.00 / kg | 10kg | 99% | 20tons | Hebei Linwo New Material Technology Co., LTD |
-

- 4-Chloro-3-methylphenol
59-50-7
- US $1.00 / kg
- 99%
- Hebei Yanxi Chemical Co., Ltd.
-

- 4-Chloro-3-methylphenol
59-50-7
- US $10.00 / kg
- 99%
- Henan Bao Enluo International TradeCo.,LTD
-

- 4-Chloro-3-methylphenol
59-50-7
- US $10.00 / kg
- 99%
- Hebei Linwo New Material Technology Co., LTD
59-50-7(4-Chloro-3-methylphenol)Related Search:
1of4