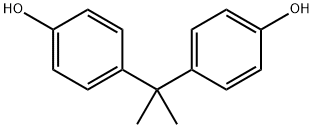
Bisphenol A
- CAS No.
- 80-05-7
- Chemical Name:
- Bisphenol A
- Synonyms
- BPA;Dian;BISPHENOL;4,4'-(propane-2,2-diyl)diphenol;Bisferol A;DIPHENYLOLPROPANE;Diano;DIANE;bisferola;Biphenol A
- CBNumber:
- CB5854419
- Molecular Formula:
- C15H16O2
- Molecular Weight:
- 228.29
- MDL Number:
- MFCD00002366
- MOL File:
- 80-05-7.mol
- MSDS File:
- SDS
| Melting point | 158-159 °C(lit.) |
|---|---|
| Boiling point | 220 °C4 mm Hg(lit.) |
| Density | 1.195 |
| vapor pressure | <1 Pa (25 °C) |
| refractive index | 1.5542 (estimate) |
| Flash point | 227 °C |
| storage temp. | Store below +30°C. |
| solubility | 0.12g/l insoluble |
| form | Liquid |
| pka | 10.29±0.10(Predicted) |
| color | Clear light yellow to light orange |
| Odor | Phenol like |
| Water Solubility | <0.1 g/100 mL at 21.5 ºC |
| Merck | 14,1297 |
| BRN | 1107700 |
| Dielectric constant | 5.0(20℃) |
| InChIKey | IISBACLAFKSPIT-UHFFFAOYSA-N |
| LogP | 3.4 at 21.5℃ |
| Indirect Additives used in Food Contact Substances | BISPHENOL A |
| FDA 21 CFR | 175.105; 175.300; 177.2280 |
| CAS DataBase Reference | 80-05-7(CAS DataBase Reference) |
| EWG's Food Scores | 2-5 |
| NCI Dictionary of Cancer Terms | BPA |
| FDA UNII | MLT3645I99 |
| Proposition 65 List | Bisphenol A (BPA) |
| NIST Chemistry Reference | Phenol, 4,4'-(1-methylethylidene)bis-(80-05-7) |
| EPA Substance Registry System | 4,4'-Isopropylidenediphenol (80-05-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS05,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H317-H318-H335-H360F-H411 | |||||||||
| Precautionary statements | P202-P273-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 37-41-43-62-52 | |||||||||
| Safety Statements | 26-36/37/39-45-46-39-36/37-61 | |||||||||
| RIDADR | UN 3077 9 / PGIII | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | SL6300000 | |||||||||
| Autoignition Temperature | 510 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29072300 | |||||||||
| Toxicity | LC50 (96 hr) in fathead minnow, rainbow trout: 4600, 3000-3500 mg/l (Staples) | |||||||||
| NFPA 704 |
|
Bisphenol A price More Price(39)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.03546 | 2,2-Bis(4-hydroxyphenyl) propane for synthesis | 80-05-7 | 100g | $35.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03546 | 2,2-Bis(4-hydroxyphenyl) propane for synthesis | 80-05-7 | 1kg | $71.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 133027 | Bisphenol A 97% | 80-05-7 | 500g | $37.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 133027 | Bisphenol A 97% | 80-05-7 | 2kg | $130 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1075892 | Bisphenol A United States Pharmacopeia (USP) Reference Standard | 80-05-7 | 200MG | $434 | 2024-03-01 | Buy |
Bisphenol A Chemical Properties,Uses,Production
Uses
A high-production-volume chemical used in manufacture of epoxy-phenolic resins (protective linings for food and beverage cans); monomer for polycarbonate resins (used in food contact materials such as returnable beverage bottles, infant feeding bottles, plates, and mugs); antioxidant in PVC plastics; inhibitor of end polymerization in PVC plastics
Description
Reports of bisphenol- A sensitization, particularly in workers at epoxy resin plants, are controversial. Bisphenol-A was also reported as an allergen in fiberglass, semisynthetic waxes, footwear and dental materials.
Chemical Properties
Bisphenol A is a white or tan crystals or flakes with a mild phenolic odor and a very low vapor pressure (ECB, 2003). It is mildly soluble in water. It is not considered to be an explosive in the conventional sense but can pose a hazard as a finely powdered material in air (ECB, 2003). It is not considered to be a chemical oxidizer.
History
Bisphenol A (BPA) was first synthesized in 1891, but it was not used widely until applications in the plastics industry were identified in the 1950s (University of Minnesota, 2008). While the most prominent use of BPA is in the manufacture of polycarbonate plastic and epoxy resins, it is also used in the production and processing of polyvinyl chloride (PVC) and modified polyamide and in the manufacture of carbonless and thermal paper, wood filler, adhesives, printing inks, surface coatings, polyurethane, brake fluid, resin-based dental composites and sealants, flame retardants, paints, and tires (ECB, 2003; EFSA, 2006).

Uses
A monomer used for policarbonate and epoxy resins; exhibits estrogenic activity. BPA is also used as a building block in polycarbonate bottles and in the epoxy-resin liners of metal cans.
Uses
Bisphenol A is used with epichlorhydrin for the synthesis of epoxy resins bisphenol-A type. It leads to bisphenol-A diglycidyl ether, which is the monomer of bisphenol-A based epoxy resins.
Uses
Bisphenol A (BPA) is used as the constitutional monomer or the monomeric building block of polycarbonate plastics, either by trans-esterification with diphenyl carbonate or via the interfacial process with a monohydroxylic phenol. Together with epichlorohydrin, BPA is also used as a major component of epoxy resins. Bisphenol A-polycarbonate plastics are in turn used in the manufacture of plastic food containers such as reusable water bottles, while epoxy resins are used as inner linings of tin cans. In addition, BPA is also used as an additive in other plastics and polymers, particularly as an antioxidant or stabilizer in polyvinyl chloride, printer ink, and in some other products.
Definition
ChEBI: A bisphenol that is 4,4'-methanediyldiphenol in which the methylene hydrogens are replaced by two methyl groups.
Preparation
The formation of bisphenol A is thought to proceed as follows:
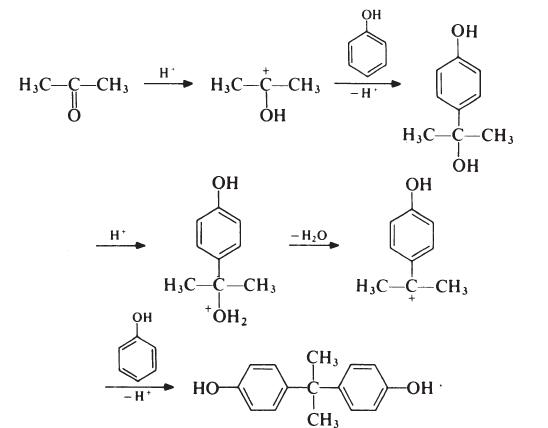
Although the reaction theoretically requires the molar ratio of reactants to be 2: 1, an improved yield of bisphenol A is obtained if additional phenol is present; the optimum molar ratio is 4: 1. In a typical process, the phenol and acetone are mixed and warmed to 50??C. Hydrogen chloride (catalyst) is passed into the mixture for about 8 hours, during which period the temperature is kept below 70??C to suppress the formation of isomeric products. Bisphenol A precipitates and is filtered off and washed with toluene to remove unreacted phenol (which is recovered). The product is then recrystallized from aqueous ethanol. Since epoxy resins are oflow molecular weight and because colour is not normally particularly important, the purity of bisphenol A used in resin production is not critical. Material with a p,p'-isomer content of 95-98% is usually satisfactory; the principal impurities in such material are o,p'- and o,o'-isomers.
Synthesis Reference(s)
Journal of the American Chemical Society, 71, p. 2287, 1949 DOI: 10.1021/ja01175a004
General Description
White to light brown flakes or powder. Has a weak medicine odor. Sinks in water.
Air & Water Reactions
The finely powdered resin is a significant dust explosion hazard. Insoluble in water.
Reactivity Profile
Bisphenol A is incompatible with strong oxidizers. Bisphenol A is also incompatible with strong bases, acid chlorides and acid anhydrides.
Hazard
Poison; moderately toxic; teratogen; irritant.
Health Hazard
Dusts irritating to upper respiratory passages; may cause sneezing.
Fire Hazard
Bisphenol A is combustible. Bisphenol A may form explosive dust clouds. Static electricity can cause its dust to explode.
Flammability and Explosibility
Not classified
Contact allergens
Bisphenol A is used with epichlorhydrin for the synthesis of epoxy resins bisphenol-A type, for unsaturated polyester and polycarbonate resins, and epoxy di(meth)acrylates. In epoxy resins, it leads to bisphenol-A diglycidyl ether, which is the monomer of bisphenol-A-based epoxy resins. Reports of bisphenol-A sensitization are rare and concern workers at epoxy resin plants, after contact with fiber glass, semi-synthetic waxes, footwear, and dental materials. It is also a possible sensitizer in vinyl gloves.
Potential Exposure
Workers engaged in the manufacture of epoxy, polysulfone, polycarbonate and certain polyester resins. It is also used in flame retardants, rubber chemicals, and as a fungicide. Bisphenol A (BP A), an environmental estrogen, is found in a wide variety of products, including polycarbonate bottles food and drink containers. According to 2008 research conducted at University of Cincinnati, when it comes to BPA, it’s not whether polycarbonate bottles are new or old but the liquid’s temperature that has the greatest impact on how much BPA is released. When exposed to boiling hot water, BPA was released 55 times more rapidly than exposure to cold water.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Environmental Fate
Bisphenol A can be released into the environment during the production, processing, and use of BPA-containing materials, although levels in environmental samples are generally very low or undetectable (ECB, 2003). This is because BPA has low volatility and a short half-life in the atmosphere, is rapidly biodegraded in water, and is not expected to be stable, mobile, or bioavailable from soils (ECB, 2003; Cousins et al., 2002).
Most environmental releases of BPA are during the manufacture of BPA-containing products when residual BPA in wastewater is released from treatment plants into receiving streams (Cousins et al., 2002). BPA's half-life in soil and water is in the order of 4.5 days while in air it is <1 day (Cousins et al., 2002). It has a low bioconcentration factor and is rapidly metabolized in fish, with a half-life of <1 day (Cousins et al., 2002).
storage
Color Code—Green: General storage may be used.Store away from heat and strong oxidizers and the incompatible materials listed above.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Crystallise bisphenol from acetic acid/water (1:1). It is used for making polycarbonate bottles and leaches out slowly on heating. It is a known “estrogenic chemical” shown to disrupt chemical signaling in the complex network of glands, hormones and cell receptors which make up the endocrine system. It causes low sperm count and damages the ecosystem by the feminisation of fish, reptiles and birds. [cf Chapter 1, p 3, Beilstein 6 IV 6717.]
Toxicity evaluation
Bisphenol-A is a chemical substance with known oestrogenic action that is used in the manufacture of a wide range of products. The low-dose in utero exposure to bisphenol-A of experimental animals caused striking morphological changes in the vagina of postpubertal offspring. In addition, the oestrogen receptor alpha was not expressed during oestrus in the vagina of female offspring exposed to bisphenol-A and the altered vaginal morphology is attributed to the down regulation of oestrogen receptor alpha (Schonfelder et at., 2002). Another experiment on mice after intrauterine exposure to bisphenol-A showed differences in the rate of ductal migration into the stroma at 1 month of age and a significant increase in the percentage of ducts, terminal ducts, terminal end buds, and alveolar buds at 6 months of age. The changes in histoarchitecture, coupled with an increased presence of secretory product within alveoli, resemble those of early pregnancy. This suggests a disruption of the hypothalamic-pituitary-ovarian axis and/or mis-expression of developmental genes. It was concluded that the altered relationship in DNA synthesis between the epithelium and stroma and the increase in terminal ducts and terminal end buds are noteworthy, because these changes are associated with carcinogenesis in both rodents and humans (Markey et at., 2001).
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid chlorides and acid anhydrides.
Bisphenol A Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29321 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7534 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 | Jany1001@kangcang.com.cn | China | 338 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 738 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
Related articles
- Biological implications of Bisphenol A
- Bisphenol A (BPA) is a chemical produced in large quantities for use primarily in the production of polycarbonate plastics and....
- Jun 23,2022
- What Is Bisphenol A and Why Is It Bad for You?
- Bisphenol A is a diphenylmethane derivative with two hydroxyphenyl groups. Bisphenol A (BISPHENOL A) is a colorless solid that....
- Feb 18,2022
- Uses of Bisphenol A
- Bisphenol A (BPA) was first synthesized in 1891, but it was not used widely until applications in the plastic industry were id....
- Nov 26,2021
View Lastest Price from Bisphenol A manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | Bisphenol A
80-05-7
|
US $2750.00-2730.00 / tons | 1tons | 99% | 1000tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-22 | Bisphenol A
80-05-7
|
US $50.00 / kg | 1kg | 99.10% | 50000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-22 | bisphenol A
80-05-7
|
US $30.00 / kg | 1kg | 98% | 20ton | Hebei Kangcang new material Technology Co., LTD |
-

- Bisphenol A
80-05-7
- US $2750.00-2730.00 / tons
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Bisphenol A
80-05-7
- US $50.00 / kg
- 99.10%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- bisphenol A
80-05-7
- US $30.00 / kg
- 98%
- Hebei Kangcang new material Technology Co., LTD