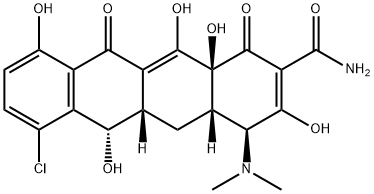
demeclocycline
- CAS No.
- 127-33-3
- Chemical Name:
- demeclocycline
- Synonyms
- D03680;Deganol;10192 RP;RP 10192;Demeclor;Mexocine;Diuciclin;Sumaclina;Ledermycin;Elkamicina
- CBNumber:
- CB5918843
- Molecular Formula:
- C21H21ClN2O8
- Molecular Weight:
- 464.85
- MDL Number:
- MFCD00864922
- MOL File:
- 127-33-3.mol
- MSDS File:
- SDS
| Melting point | 176°C (rough estimate) |
|---|---|
| Boiling point | 787.1±60.0 °C(Predicted) |
| Density | 1.3118 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| solubility | H2O:1.5(Max Conc. mg/mL);3.23(Max Conc. mM) |
| pka | 4.50±1.00(Predicted) |
| Water Solubility | 1.4g/L(25 ºC) |
| FDA UNII | 5R5W9ICI6O |
demeclocycline Chemical Properties,Uses,Production
Description
Demethylchlortetracycline was isolated from the culture broth of a mutant of Streptomyces aureofaciens, the chlortetracycline-producing strain, by Lederle Research Laboratories in 1957. It shows one and one-half to two times as much in vitro antimicrobial activity and in vivo protective effect as tetracycline. Its base and hydrochloride have been used orally and by topical application to treat infections caused by Staphylococcus, Streptococcus, Rickettsia, Chlamydiae, Neisseria, Klebsiella, Proteus, Escherichia coli, and Haemophilus influenzae.
Originator
Declomycin,Lederle,US,1959
Uses
Demeclocycline, a chlortetracycline analogue produced by a mutagenised strain of Streptomyces aureofaciens, was first isolated in 1957. Like all tetracyclines, demeclocycline shows broad spectrum antibacterial and antiprotozoan activity and acts by binding to the 30S and 50S ribosomal subunits, blocking protein synthesis. Demeclocycline has been extensively cited in the literature with over 800 references relating almost exclusively to in vivo use.
Definition
ChEBI: Tetracycline which lacks the methyl substituent at position 7 and in which the hydrogen para- to the phenolic hydroxy group is substituted by chlorine. Like tetracycline, it is an antibiotic, but being excreted more slowly, effective blood lev ls are maintained for longer. It is used (mainly as the hydrochloride) for the treatment of Lyme disease, acne and bronchitis, as well as for hyponatraemia (low blood sodium concentration) due to the syndrome of inappropriate antidiuretic hormone (SIADH) w ere fluid restriction alone has been ineffective.
Manufacturing Process
According to US Patent 2,878,289, a suitable medium for the preparation of
inocula for the fermentation may be prepared with the following substances.
The pH of the medium thus prepared is about 6.8. An 8 ml portion is
measured into an 8 inch Brewer tube and sterilized at 120°C for 20 minutes.
The sterilized medium is then inoculated with 0.5 ml of an aqueous spore
suspension of a strain of S. aureofaciens capable of producing
chlorodemethyltetracycline, such as S-604, containing approximately 40-60
million spores per milliliter. The inoculated medium is incubated for 24 hours
at 28°C on a reciprocating shaker operated at 110 cycles per minute.
A suitable fermentation medium contains water and a source of assimilable
carbon and nitrogen and essential mineral salts. A typical medium suitable for
production of chlorodemethyltetracycline is as follows:
A suitable fermentation medium contains water and a source of assimilable
carbon and nitrogen and essential mineral salts. A typical medium suitable for
production of chlorodemethyltetracycline is as follows:
Ten percent of the resulting inoculum is then transferred to a 250 ml
Erlenmeyer flask containing 50 ml of the medium employed above and the
flask agitated a further 72 hours under the same conditions. One ml of the
resulting inoculum is then employed for the inoculation of 10 ml of an
aqueous medium containing, per 1,000 ml, 30 grams extraction process
soybean meal, 1 gram sodium chloride, 50 grams glucose and 7 grams
calcium carbonate, in a 1" x 6" test tube.
In addition, 1 mg of sterile S-2-hydroxyethyl-DL-homocysteine is added to the
tube and the tube is shaken on a rotary shaker at 280 cycles per minute at
25°C for seven days. The contents of the tube were then acidified to pH 2 by
the addition of sulfuric acid and centrifuged. Examination of the supernatant
liquid by paper chromatography employing the methods of Bohonos et al,
Antibiotics Annual (1953-4, page 49),demonstrates the presence of 7-chloro6-demethyltetracycline,7-chlorotetracycline and tetracycline.
brand name
Declomycin (Lederle).
Therapeutic Function
Antibacterial
Safety Profile
Poison by intravenous and intraperitoneal routes. Human systemic effects by ingestion: diabetes insipidus, urine volume increase, other changes in urine composition, dermatitis, changes in the nails, allergic rhinitis, serum sickness, effects on cyclic nucleotides. Human reproductive effects by an unspecified route: postnatal measures or effects on newborn. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic fumes of Cland NOx.
demeclocycline Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| BOC Sciences | 1-631-485-4226; 16314854226 | info@bocsci.com | United States | 14059 | 65 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12338 | 58 |