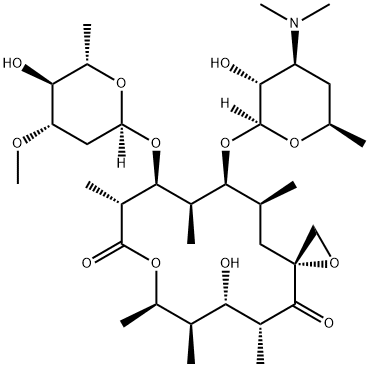
oleandomycin
- CAS No.
- 3922-90-5
- Chemical Name:
- oleandomycin
- Synonyms
- C01946;PA 775;PA-105;Romicil;Amimycin;Landomycin;oleandomycin;OleandoMycin A;Antibiotic PA-105;AMiMycin, MatroMycin, PA 105
- CBNumber:
- CB5928730
- Molecular Formula:
- C35H61NO12
- Molecular Weight:
- 687.86
- MDL Number:
- MFCD00242812
- MOL File:
- 3922-90-5.mol
- MSDS File:
- SDS
| Melting point | 110 °C (decomp) |
|---|---|
| Boiling point | 802.6±65.0 °C(Predicted) |
| Density | 1.21±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 8.84(H2O,t =25,I=0.167) (Uncertain) |
| color | White to Off-White |
| EWG's Food Scores | 1 |
| FDA UNII | P8ZQ646136 |
| ATC code | J01FA05 |
oleandomycin price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 16552 | Oleandomycin ≥95% | 3922-90-5 | 5mg | $121 | 2024-03-01 | Buy |
| Cayman Chemical | 16552 | Oleandomycin ≥95% | 3922-90-5 | 25mg | $423 | 2024-03-01 | Buy |
| Usbiological | 257360 | Oleandomycin | 3922-90-5 | 1mg | $744 | 2021-12-16 | Buy |
| TRC | O517500 | Oleandomycin | 3922-90-5 | 1mg | $140 | 2021-12-16 | Buy |
| TRC | O517500 | Oleandomycin | 3922-90-5 | 2.5mg | $305 | 2021-12-16 | Buy |
oleandomycin Chemical Properties,Uses,Production
Originator
Matromycin,Pfizer,US,1956
Uses
Oleandomycin is a 16-membered macrocyclic lactone, discovered in the 1950s, with broad spectrum antibacterial activity. Oleandomycin was developed as a human pharmaceutical but was regarded as less active than comparable products such as erythromycin and is today only available in combination with other antibiotics.
Uses
Antibiotic substance produced by Streptomyces antibioticus no. ATCC 11891. Antibacterial.
Definition
ChEBI: Oleandomycin is a member of oleandomycins. It is functionally related to an oleandolide. It is a conjugate base of an oleandomycin(1+).
Manufacturing Process
A slant of S. antibioticus ATCC 11891 was cultivated on agar under controlled conditions in order to develop spores for the purpose of inoculating a nutrient medium having the following composition: 20 g Cerelose (dextrose hydrate), 15 g soybean meal, 5 g distillers' solubles, 10 g cornmeal, and tap water, in a sufficient amount for a 1,000 ml solution, adjusted to pH 7.0 to 7.2 with potassium hydroxide.
After the pH was adjusted, 5 g of calcium carbonate was added. This inoculum medium was then subjected to heat sterilization. The medium was then cooled and 2 ml of a spore suspension of an oleandomycin-producing strain of S. antibioticus was added under aseptic conditions. The cultivation of the organism was conducted in shaken flasks at 28% for a period of 48 hours.
The mixture of broth and mycelium thus formed was then transferred under aseptic conditions to a 3-liter fermentor containing 2,000 ml of a sterile
fermentation medium having the following composition: 60 g Cerelose (dextrose hydrate), 18 g soybean meal, 5 g distillers' solubles, 12 g cornmeal and tap water in a sufficient amount for a 1,000 ml total volume, adjusted to pH 7.0 to 7.2 with potassium hydroxide.
After the pH had been adjusted, 5 g of calcium carbonate, 5 ml of soybean oil antifoam and 0.020 g of Acridine Orange dye were added. The mixture was then autoclaved at 20 psi (250°F) for 15 minutes in order to sterilize the contents, before transferring the broth and mycelium thereto.
After seeding the nutrient medium with the preformed inoculum previously described, the mixture was subjected to agitation and aeration under aseptic conditions for 72 hours; at 27°C to 28°C for the first 24 hours, then at 25°C to 26°C for the next 48 hours; during this period, the pH was in the range of 6.4 to 6.8. Aeration was accomplished by cultivation under submerged conditions at an air flow rate of one volume of air per volume of medium per minute. After termination of the process, the mycelium was removed by filtration and the filtered broth found to contain 450 γ of oleandomycin per ml of solution.
brand name
Matromycin (Pfizer).
Therapeutic Function
Antibiotic
Pharmaceutical Applications
A natural 14-membered-ring macrolide produced by Streptomyces antibioticus. It is stable in acid conditions. It is less active than erythromycin A in vitro, but four times more active than spiramycin . Several attempts have been made to improve its potency by chemical modification while retaining its relative acid stability.
It is incompletely absorbed, but an ester, triacetyloleandomycin, gives improved plasma levels. Following doses of 0.5 g, mean peak serum levels around 0.8 mg/L were reached by the base and 2 mg/L by the triacetyl ester. A single oral dose of 1 g of the ester produced a mean plasma oleandomycin concentration of 4 mg/L at 1 h after dosing, with an AUC of 14 mg.h/L. The apparent elimination half-life was 4.2 h. Significant quantities of mostly inactivated compound are eliminated in the bile. About 10% of the dose appears in the urine after administration of the base and about 20% after the ester.
Nausea, vomiting and diarrhea are common. Like erythromycin estolate, triacetyloleandomycin can cause liver damage. Abnormal liver function tests were found in about one-third of patients treated for 2 weeks. Hepatic dysfunction resolved when treatment was discontinued. The action of drugs eliminated via the cytochrome P450 system may be potentiated.
Its uses are similar to those of erythromycin. It is of restricted availability.
Biological Activity
oleandomycin is an antibiotic produced by strains of streptomyces.
Safety Profile
A poison by intravenous route. Low toxicity by ingestion. When heated to decomposition it emits toxic vapors of NOx.
in vitro
oleandomycin has been identified as a macrolide antibiotic demonstrating antimicrobial activity similar to penicillin and erythromycin. in addition, oleandomycin structurally has a macrocyclic lactone ring of 14 carbon atoms with one sugar, oleandrose, and one amino sugar, desoxamine [1].
in vivo
the pk of oleandomycin after i.v. and po administration, both alone and after i.m. pretreatment with metamizole or dexamethasone, was studied in healthy dogs. results showed that after i.v. injection of oleandomycin alone at 10 mg/kg, the t1/2β, vd, cl and auc were 1.60 h, 1.11 l/kg, 7.36 ml/kg/min and 21.66 μg h/ml, respectively. there were no statistically significant differences following pretreatment with metamizole or dexamethasone. in addition, after po administration of oleandomycin alone, the t1/2β, cmax, tmax), mat and fabs were 1.68 h, 5.34 μg/ml, 1.5 h, 1.34 h and 84.29%, respectively, and the pretreatment with metamizole showed a significantly decreased value for cmax but the mat was increased significantly. statistically significant changes in the pk parameters of oleandomycin following po administration were also seen as a result of pretreatment with dexamethasone. the cmax was increased and the tmax and mat were lower [2].
References
[1] c. vilches, c. méndez, c. hardisson, et al. biosynthesis of oleandomycin by streptomyces antibioticus: influence of nutritional conditions and development of resistance. j.gen.microbiol. 136(8), 1447-1454 (1990).
[2] milanova a, lashev l. pharmacokinetics of oleandomycin in dogs after intravenous or oral administration alone and after pretreatment with metamizole or dexamethasone. vet res commun. 2002 jan;26(1):61-71.
oleandomycin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | contact@fuxinpharm.com | China | 10297 | 58 |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | contact@trustwe.com | China | 5738 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Zhejiang Huida Biotech Co., LTD | 008613515763466 8615669048680 | wendy@huidabiotech.com | CHINA | 87 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Chemsky(shanghai)International Co.,Ltd. | 021-50135380 | shchemsky@sina.com | China | 32344 | 50 |