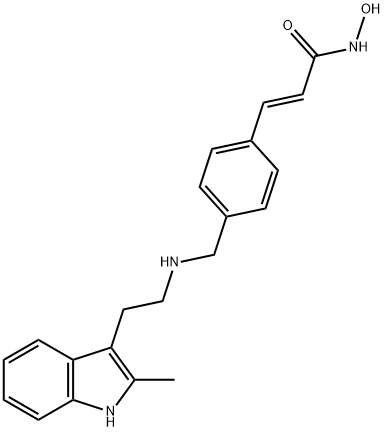
Panobinostat
- CAS No.
- 404950-80-7
- Chemical Name:
- Panobinostat
- Synonyms
- CS-422;LBH-589;NVP-LBH589;Palcociclib;panobinosta;PANOBINOSTAT;2-Acrylamide;Palmer than he;Panobinostat D7;Panobinostat, >=98%
- CBNumber:
- CB61009161
- Molecular Formula:
- C21H23N3O2
- Molecular Weight:
- 349.43
- MDL Number:
- MFCD09833242
- MOL File:
- 404950-80-7.mol
| Melting point | 114-117?C |
|---|---|
| Density | 1.241±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | Soluble in DMSO (up to 100 mg/ml) or in Ethanol (up to 5 mg/ml with warming). |
| form | solid |
| pka | 8.71±0.23(Predicted) |
| color | Off-white |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. |
| NCI Dictionary of Cancer Terms | LBH589; panobinostat |
| FDA UNII | 9647FM7Y3Z |
| ATC code | L01XH03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 | |||||||||
| Risk Statements | 22-36/37/38-20 | |||||||||
| Safety Statements | 24/25 | |||||||||
| HS Code | 29339900 | |||||||||
| NFPA 704 |
|
Panobinostat price More Price(34)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 13280 | Panobinostat ≥98% | 404950-80-7 | 5mg | $29 | 2024-03-01 | Buy |
| Cayman Chemical | 13280 | Panobinostat ≥98% | 404950-80-7 | 10mg | $55 | 2024-03-01 | Buy |
| Cayman Chemical | 13280 | Panobinostat ≥98% | 404950-80-7 | 50mg | $99 | 2024-03-01 | Buy |
| Cayman Chemical | 13280 | Panobinostat ≥98% | 404950-80-7 | 25mg | $147 | 2021-12-16 | Buy |
| Usbiological | 170036 | Panobinostat | 404950-80-7 | 1mg | $293 | 2021-12-16 | Buy |
Panobinostat Chemical Properties,Uses,Production
Description
Panobinostat is a potent inhibitor of all histone deacetylases (HDACs), with Ki values ranging from 0.6 to 31 nM for HDAC1-
Chemical Properties
Yellow Solid
Uses
The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells.
Uses
The novel labelled histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells.
Definition
ChEBI: A hydroxamic acid obtained by formal condensation of the carboxy group of (2E)-3-[4-({[2-(2-methylindol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enoic acid with the amino group of hydroxylamine. A histone deacetylase inhibitor used (as its la tate salt) in combination with bortezomib and dexamethasone for the treatment of multiple myeloma.
Clinical Use
Histone deacetylase (HDAC) inhibitor:
Treatment of relapsed and/or refractory multiple
myeloma
target
HDAC (MOLT-4 cells)
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: avoid with dextromethorphan possibly
increased risk of ventricular arrhythmias with
methadone - avoid.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with amiodarone and possibly
disopyramide - avoid.
Antibacterials: increased risk of ventricular
arrhythmias with clarithromycin and moxifloxacin -
avoid; avoid with rifampicin.
Antidepressants: avoid with St John’s wort.
Antiepileptics: avoid with carbamazepine,
fosphenytoin, phenobarbital, phenytoin and
primidone.
Antifungals: concentration increased by ketoconazole
and possibly posaconazole and voriconazole - reduce
panobinostat dose; concentration possibly increased
by itraconazole - avoid.
Antimalarials: possibly increased risk of ventricular
arrhythmias with chloroquine - avoid.
Antipsychotics: avoid with pimozide.
Antivirals: concentration possibly increased
by ritonavir and saquinavir - reduce dose of
panobinostat.
Beta-blockers: possibly increased risk of ventricular
arrhythmias with sotalol - avoid.
Grapefruit juice: avoid concomitant use.
5HT3
antagonists: possibly increased risk of
ventricular arrhythmias with granisetron and
ondansetron - avoid with granisetron.
Metabolism
Panobinostat is extensively metabolised, and a large
fraction of the dose is metabolised before reaching
the systemic circulation by reduction, hydrolysis,
oxidation and glucuronidation. Oxidative metabolism
of panobinostat played a less prominent role, with
approximately 40% of the dose eliminated by this
pathway. Cytochrome P450 3A4 (CYP3A4) is the main
oxidation enzyme, with potential minor involvement of
CYP2D6 and 2C19.
Panobinostat represented 6-9% of the drug-related
exposure in plasma. The parent substance is deemed to
be responsible for the overall pharmacological activity of
panobinostat.
After a single oral dose of [14C]-panobinostat in patients,
29-51% of administered radioactivity is excreted in the
urine and 44-77% in the faeces. Unchanged panobinostat
accounted for <2.5% of the dose in urine and <3.5% of
the dose in faeces.
storage
Store at -20°C
References
1) Geng et al. (2006), Histone deacetylase (HDAC) inhibitor LBH589 increases duration of gamma H2AX foci and confines HDAC4 to the cytoplasm in irradiated non-small cell lung cancer; Cancer Res., 66 11298 2) George et al. (2005), Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3; Blood, 105 1768 3) Maiso et al. (2006), The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance; Cancer Res., 66 5781
Panobinostat Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
View Lastest Price from Panobinostat manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-12 | Panobinostat
404950-80-7
|
US $0.00 / kg | 1kg | 99% | 2000ton | Shaanxi Haibo Biotechnology Co., Ltd | |
 |
2023-09-15 | Panobinostat
404950-80-7
|
US $10.00-1.00 / kg | 1kg | 0.99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2022-10-18 | Panobinostat
404950-80-7
|
US $50.00 / kg | 1kg | 99% | 1 | Hebei Duling International Trade Co. LTD |
-

- Panobinostat
404950-80-7
- US $0.00 / kg
- 99%
- Shaanxi Haibo Biotechnology Co., Ltd
-

- Panobinostat
404950-80-7
- US $10.00-1.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Panobinostat
404950-80-7
- US $50.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD