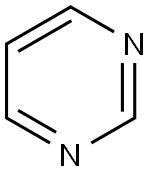
Pyrimidine
- CAS No.
- 289-95-2
- Chemical Name:
- Pyrimidine
- Synonyms
- Pyrimidin;1,3-DIAZINE;Metadiazine;2,4,6-TRICHLORO-5-FLUOROPYRIMIDINE;2,4,6-trichloro-5-formylpyrimidine;iazine;Miazine;m-Diazine;PYRIMIDINE;1,3-Diazin
- CBNumber:
- CB6224079
- Molecular Formula:
- C4H4N2
- Molecular Weight:
- 80.09
- MDL Number:
- MFCD00006059
- MOL File:
- 289-95-2.mol
- MSDS File:
- SDS
| Melting point | 19-22 °C (lit.) |
|---|---|
| Boiling point | 123-124 °C (lit.) |
| Density | 1.016 g/mL at 25 °C (lit.) |
| refractive index |
n |
| Flash point | 88 °F |
| storage temp. | Flammables area |
| solubility | soluble |
| form | Liquid |
| pka | 1.23(at 20℃) |
| color | Clear colorless to orange |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,7987 |
| BRN | 103894 |
| Stability | Stable, but air-sensitive and hygroscopic. Incompatible with acids, acid chlorides, acid anhydrides, strong oxidizing agents, carbon dioxide. Flammable. |
| CAS DataBase Reference | 289-95-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | pyrimidine |
| FDA UNII | K8CXK5Q32L |
| NIST Chemistry Reference | 1,3-Diazine(289-95-2) |
| EPA Substance Registry System | Pyrimidine (289-95-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P242-P243 | |||||||||
| Risk Statements | 10 | |||||||||
| Safety Statements | 23-24/25-16 | |||||||||
| RIDADR | UN 1993 3/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UV6263000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29335990 | |||||||||
| NFPA 704 |
|
Pyrimidine price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 131695 | Pyrimidine ≥98.0% | 289-95-2 | 1g | $45.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 131695 | Pyrimidine ≥98.0% | 289-95-2 | 5g | $124 | 2024-03-01 | Buy |
| TCI Chemical | P0662 | Pyrimidine free base >98.0%(GC)(T) | 289-95-2 | 1g | $33 | 2024-03-01 | Buy |
| TCI Chemical | P0662 | Pyrimidine free base >98.0%(GC)(T) | 289-95-2 | 5g | $91 | 2024-03-01 | Buy |
| Alfa Aesar | A15534 | Pyrimidine, 99% | 289-95-2 | 1g | $31.65 | 2024-03-01 | Buy |
Pyrimidine Chemical Properties,Uses,Production
Six-membered heterocyclic compounds
Pyridine, also known as "metadiazine", is a six-membered heteroaromatic ring compound containing two nitrogen atoms in the meta-position, being isomers with pyridazine and pyrazine. It has a molecular formula of C4H4N2 with the molecular weight being 80.09. It appears as colorless liquid or solid crystals with a pungent odor. It has a melting temperature of 20~22 ℃, boiling point of 123~124 °C and refractive index of 1.4998 (20 °C). It is easily soluble in water, ethanol and ether with weak alkaline and being capable of forming salts with acid. Its alkalinity is weaker than pyridine. It is also more difficult for it to participate into electrophilic substitution reaction than pyridine. It can only undergo bromination at the 5th position and is not capable of having nitrification and sulfonation reaction, but more prone to participate into nucleophilic substitution. Pyrimidine derivatives are widely found in nature. For example, vitamin B1, uracil, cytosine and thymine all contain pyrimidine structure. Its picrate salt appears as yellow needle-like crystals. It has a melting temperature of 156 °C. Owing to the presence of conjugated double bonds in the structure, pyrimidine has strong absorption capability on ultraviolet light. It is manufactured through phosphorus oxychloride oxidation of barbituric acid, followed by reduction through hydrogen iodide.
Nucleic acid contains three important pyrimidine derivatives, being an important base in nucleic acid, plays an important role in the body of organisms.
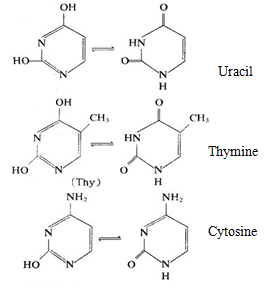
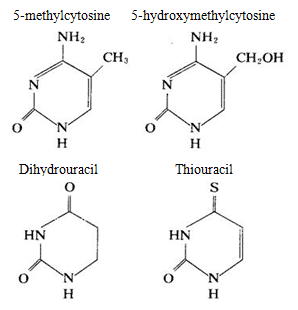
DNA mainly contains cytosine and thymine while RNA mainly contains cytosine and uracil, in some nucleic acids, there are also small amount of pyrimidine modified base, for example:
Sulfadiazine and its derivatives are commonly used antibacterial anti-inflammatory drugs.
The above information is edited by Andy Edwards of Chemicalbook.
Chemical properties
It appears as colorless crystalline or liquid.
Uses
Pyrimidine is a heterocyclic organic molecule found in numerous pharmaceutical and naturally occurring compounds. It plays a significant role in the synthesis and discovery of antiviral medications, including those targeting HIV and HSV. Additionally, pyrimidine is involved in the synthesis of potent 15-lipoxygenase inhibitors that help reduce the release of leukotrienes. While pyrimidine itself has no direct applications, its derivatives, cytosine, uracil, and thymine, are essential components of nucleic acids. Various drugs, such as sulfadiazine, trimethoprim, and 6-mercaptopurine, also contain a pyrimidine ring.
Pyrimidine base
Pyrimidine base is one of the chemical compositions of the nucleotides. It consists of carbon, nitrogen, hydrogen, oxygen and other elements. Pyrimidine bases include uracil, cytosine, and thymine, where uracil and cytosine constitute the bases in ribonucleic acid molecules while thymine and cytosine constitute bases in deoxyribonucleic acid molecules. Pyrimidine base has a strong absorption on ultraviolet in the wavelength of 250~280nm. The raw materials for its synthesis are derived from carbamoyl phosphate and aspartic acid. Pyrimidine base can also be metabolized into carbon dioxide, β-alanine and β amino-isobutyric acid and other substances. Some patients, due to congenital factors or taking certain drugs, can get pyrimidine alkali metabolic disorders, causing whey aciduria.
The functions of purine, pyrimidine and other substances
Purines and pyrimidines are essential heterocyclic nitrogen-containing compounds that used in nucleic acid metabolism in organisms (including humans) and are important substances in the formation of ribonucleic acid and deoxyribonucleic acid in cells. Purine, pyrimidine and ribose and phosphate can combine to form RNA; purine, pyrimidine and deoxyribose can bind to phosphate to produce DNA. DNA is the main chemical constituent of genes, which plays an important role in the transmission of genes (genetics); the main function of RNA is the regulation of intracellular protein synthesis. The final product of purine metabolism is mainly uric acid. Barley and malt contains 0.2% to 0.3% of the dry matter of nucleic acid. Upon the saccharification, the dry matter of nucleic acid can be subject to the degradation of various phosphatases to form nucleotides, nucleosides, purines and pyrimidine and many other degradation products, of which only purine and pyrimidine can enter into yeast cells to constitute ribonucleic acid, deoxyribonucleic acid, adenosine triphosphate and some coenzymes. Nucleotides are difficult to be absorbed. If the medium is lack of purine and pyrimidine, the cells have to rely on carbohydrates and amino acids for synthesis, thus consuming a lot of energy and influence the proliferation of yeast. In general, wort is not lack of the required purine and pyrimidine.
References: Chinese Medical Encyclopedia Editorial Board Editor; Guo Di editor in chief. Chinese Encyclopedia of Medicine • fifty-seven pediatrics.
Preparation
1, take malondialdehyde and formamide as raw materials and have reaction upon heating; we can obtain pyrimidine.
2, it can be manufactured through the catalytic hydrogenation of 2, 4-dichloropyrimidine in the system.
3, use zinc powder to reduce 2, 4, 6-trichloropyrimidine, pyrimidine can be obtained.
4, the reaction between ethyl acetoacetate and amidine (below) can produce pyrimidine.
Fluorouracil
Fluorouracil is a pyrimidine antimetabolite. In the body, it is first converted into 5-fluoro-2-deoxyuridine nucleotides (FUdRP), causing inhibition of thymidylate synthase (TMPS), preventing the conversion of deoxyuridine nucleotide into thymidine, interfering with DNA synthesis and leading to cell damage and death. It has a stronger effect in the presence of leucovorin (CF) because FUdRP, FH4 and TMPS can form triple complexes making the active metabolite of drug bind more tightly with the enzyme, so addition of CF when applying will yield better efficacy, especially improving the efficacy upon being applied to colorectal cancer. This product is cell cycle-specific drugs with killing effects on proliferated cells at all stages. It is most sensitive to the S phase, and also has retardation effect on the G1/S border. Oral absorption is not complete, and the drug is easily inactivated upon liver metabolism. After intravenous infusion or arterial infusion, the blood concentration is relatively stable.
Fluorouracil has relative significant effect against choriocarcinoma and malignant mole. It also has certain curative effects on gastric cancer, colon cancer, rectum cancer, liver cancer, pancreatic cancer, breast cancer, ovarian cancer, cervical cancer, prostate cancer, bladder cancer, kidney cancer, lung cancer, head and neck cancer and skin cancer.
Chemical Properties
Colourless to pale yellow liquid
Uses
Pyrimidine is used as building block in chemical synthesis. Product Data Sheet
Uses
Pyrimidine was used to assess the extent of pyrimidine/purine asymmetry quantitatively. It was also used to study the photoinduced ion chemistry of the halogenated pyrimidines, a class of prototype radiosensitizing molecules.
Definition
ChEBI: Pyrimidine is a simple nitrogenous organic molecule whose ring structure is contained in the pyrimidine bases cytosine, thymine, and uracil, which are constituents of the nucleic acids. It has a role as a Daphnia magna metabolite. It is a member of pyrimidines and a diazine.
Pyrimidine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Xinxiang Hongqi District Houyuan Trading Co.,Ltd | +86-0373-3695376 +86-13937349994 | HYJM@houyuanjm.com | China | 300 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 424 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
Related articles
- What is a pyrimidine made of?
- Pyrimidine is a ring structure composed of four carbon atoms and two nitrogen atoms.
- Nov 9,2022
View Lastest Price from Pyrimidine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-19 | Pyrimidine
289-95-2
|
US $6.00 / KG | 1KG | More than 99% | 2000KG/Month | Hebei Saisier Technology Co., LTD | |
 |
2023-12-25 | Pyrimidine
289-95-2
|
US $0.00 / kg | 1kg | 98% | 1000000 | Hebei Jingbo New Material Technology Co., Ltd | |
 |
2023-09-15 | Pyrimidine
289-95-2
|
US $100.00 / drum | 1drum | 99 | 5000 | Hebei Fengqiang Trading Co., LTD |
-

- Pyrimidine
289-95-2
- US $6.00 / KG
- More than 99%
- Hebei Saisier Technology Co., LTD
-

- Pyrimidine
289-95-2
- US $0.00 / kg
- 98%
- Hebei Jingbo New Material Technology Co., Ltd
-

- Pyrimidine
289-95-2
- US $100.00 / drum
- 99
- Hebei Fengqiang Trading Co., LTD
289-95-2(Pyrimidine)Related Search:
1of4