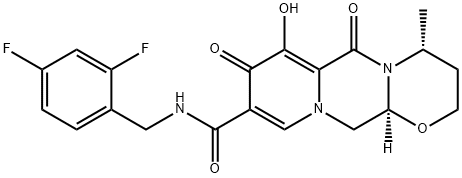
GSK1349572
- CAS No.
- 1051375-16-6
- Chemical Name:
- GSK1349572
- Synonyms
- Dolutegravir;Dolutegravi;S/GSK1349572;Dulutegravir;Dolutegravir (GSK1349572);CS-9;134413;S-349572;GSK1349572;BNKY001-DL04
- CBNumber:
- CB62538916
- Molecular Formula:
- C20H19F2N3O5
- Molecular Weight:
- 419.38
- MDL Number:
- MFCD20488027
- MOL File:
- 1051375-16-6.mol
- MSDS File:
- SDS
| Melting point | 187-189°C |
|---|---|
| Boiling point | 669.0±55.0 °C(Predicted) |
| Density | 1.53 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Heated, Sonicated) |
| pka | 4.50±1.00(Predicted) |
| form | Solid |
| color | White to Pale Beige |
| FDA UNII | DKO1W9H7M1 |
| NCI Drug Dictionary | dolutegravir |
| ATC code | J05AJ03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| NFPA 704 |
|
GSK1349572 price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 22191 | Dolutegravir ≥98% | 1051375-16-6 | 5mg | $117 | 2024-03-01 | Buy |
| Cayman Chemical | 22191 | Dolutegravir ≥98% | 1051375-16-6 | 10mg | $222 | 2024-03-01 | Buy |
| Cayman Chemical | 22191 | Dolutegravir ≥98% | 1051375-16-6 | 25mg | $521 | 2024-03-01 | Buy |
| Cayman Chemical | 22191 | Dolutegravir ≥98% | 1051375-16-6 | 50mg | $866 | 2024-03-01 | Buy |
| TRC | D528800 | Dolutegravir | 1051375-16-6 | 10mg | $165 | 2021-12-16 | Buy |
GSK1349572 Chemical Properties,Uses,Production
Anti-AIDS drugs
Dolutegravir (Tivicay) was a new kind of anti-ADIS drug that jointly developed by the British pharmaceutical giant GlaxoSmithKline (GSK) with the Japanese Shionogi Pharmaceutical Company (Shionogi).
In July 2012, the GlaxoSmithKline Pharmaceuticals and Japan's Shionogi Pharmaceutical Company announced the results of Phase III clinical trial of the new AIDS drug Dolutegravir. After 48 weeks of treatment with dolutegravir and two other older versions of the AIDS drug, 88% of the virus in vivo was successfully inhibited, while the use of Gilead Sciences (Gilead Sciences) of the three-in-one oral drug Atripla (Efavirenz/Emtricitabine/Tenofovir Disoproxil Fumarate), 81% of the virus in patients was inhibited, which can be seen that, the dolutegravir developed by the GlaxoSmithKline pharmaceutical companies is slightly better. According to the researchers, in a comparative trial, owing to the side effects of the drugs, 10% of the patients stopped taking the Atripla drug developed from Gilead Technologies while only 2% stopped taking GlaxoSmithKline's dolutegravir Drug, therefore, we can see that the dolutegravir drugs from GlaxoSmithKline has slightly higher safety.
On August 12, 2013, the US Food and Drug Administration (FDA) approved the use of dolutegravir for being used in previously treated or early treated HIV-1 adults and 12 years of age and above infected children of at least 40 kg.
Dolutegravir is a once-daily drug with its efficacy being comparable to Merck's HIV/AIDS drug Raltegravir (Isentress) in Phase III clinical trials. Raltegravir should be subject to daily administration twice. Both of them are inhibitors of HIV integrase. The FDA official claim that the AIDS patient should be subject to targeted treatment on a case-by-case basis. Tivicay will provide patients with new options. In a study done a year ago, 88% of patients had a significant improvement after 48 weeks of Tivicay treatment, better than the efficacy of the Gilead's Atripla. Analysts expect that Dolutegravir will become a multi-billion-dollar blockbuster drug and a strong contender for Atripla, the world's best-selling HIV drug, developed by Gilead Sciences.
Common name: Dolutegravir
Trade name: Tivicay
Alias: GSK1349572, S-349572, GSK572
Drug Company: GlaxoSmithKline
Indications: AIDS
Drug type: integrase inhibitors
Approved date: August 12, 2013 (US)
CAS Registry Number: 1051375-16-6
Chemical name: (4R, 12aS)-N-[(2, 4-difluorophenyl) methyl]-3, 4, 6, 8, 12, 12a-hexahydro-7-hydroxy-Dioxo-2H-pyrido [1 ', 2': 4,5] pyrazino [2,1-b] [1,3] oxazine-9-carboxamide
U.S. Patent No. 8,129,385
The patent expires on October 5, 2027
International patent: W02006116764
This information is compiled and edited by Xiao Nan of Chemicalbook.
Uses
Dolutegravir (GSK1349572) is a HIV integrase inhibitor with an IC50 of 2.7 nM and is moderately effective against the significant mutants Y143R, Q148K, N155H, and G140S/Q148H against Raltegravir.
Description
In August 2013, the US FDA approved dolutegravir (also referred to as S/GSK1349572) for the treatment of HIV-1 infection in adults and children ages 12 years and older in combination with other antiretroviral drugs. Dolutegravir was approved in Canada in November 2013. HIV/AIDS remains a global epidemic with 35 million people infected, including 2.3 million new infections as of 2012. Dolutegravir joins raltegravir and elvitegravir (this chapter of ARMC) as the latest of three FDA-approved HIV integrase strand transfer inhibitors (INSTIs). Dolutegravir was discovered by rational design from a literature diketo acid HIV integrase inhibitor utilizing X-ray coordinates to predict ideal bond angles between the diketone and distal benzyl group. In dolutegravir, the monocyclic component of the reported inhibitor was replaced with the tricyclic carbamoyl pyridone moiety. The researchers postulated that the appropriate arrangement of three oxygens would permit chelation with two magnesium ions in the binding site thus affording improved potency. Ultimately, this arrangement along with further modifications afforded dolutegravir, a potent inhibitor of HIV integrase (IC50=1.7 nM).
Chemical Properties
White Solid
Originator
Shionogi & GlaxoSmithKline (United States)
Uses
Dolutegravir is a second generation HIV-1 integrase strand transfer inhibitor. Dolutegravir is currently in Phase III clinical trials for the treatment of HIV infection. Dolutegravir has been shown to potently inhibit HIV replication in cells such as peripheral blood mononuclear cells (PBMCs), MT-4 cells and CIP4 cells infected with a self-inactivating PHIV lentiviral vector.
Uses
Dolutegravir is a second generation HIV-1 integrase strand transfer inhibitor. Dolutegravir is currently in Phase III clinical trials for the treatment of HIV infection. Dolutegravir has been shown to potently inhibit HIV replication in cells such as peripheral blood mononuclear cells (PBMCs), MT-4 cells and CIP4 cells infected with a self-inactivating PHIV lentiviral vector.
Definition
ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of (4R,12aS)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]ox zine-9-carboxylic acid with the amino group of 2,4-difluorobenzylamine. Used (as its sodium salt) for treatment of HIV-1.
brand name
Tivicay
Clinical Use
Integrase inhibitor:
Treatment of HIV
target
HIV integrase
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: concentration reduced by St John’s
wort.
Antiepileptics: concentration reduced by
carbamazepine and possibly fosphenytoin,
oxcarbazepine, phenobarbital, phenytoin and
primidone.
Antivirals: concentration reduced by efavirenz,
tipranavir, etravirine and fosamprenavir; possibly
reduced by nevirapine.
Metabolism
Dolutegravir is primarily metabolised through
glucuronidation via UGT1A1 with a minor CYP3A
component.
53% of the total oral dose is excreted unchanged in
the faeces. It is unknown if all or part of this is due to
unabsorbed active substance or biliary excretion of the
glucuronidate conjugate, which can be further degraded to
form the parent compound in the gut lumen.
GSK1349572 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Guangzhou Tengyue Chemical Co., Ltd. | +86-86-18148706580 +8618826483838 | evan@tyvovo.com | China | 152 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9352 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Lianyungang happen teng technology co., LTD | 15950718863 | wang666xt@163.com | CHINA | 295 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
View Lastest Price from GSK1349572 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-01-04 | Dolutegravir
1051375-16-6
|
US $120.00-80.00 / g | 1g | 98%HPLC | 1T | Shenzhen Nexconn Pharmatechs Ltd | |
 |
2023-11-16 | Dolutegravir; DTG; GSK1349572
1051375-16-6
|
US $240.00 / g | 10g | 99% Purity (What/sapp: +86 18145728414) | 1000 Tons/Month | Guangzhou Tengyue Chemical Co., Ltd. | |
 |
2021-11-25 | GSK1349572
1051375-16-6
|
US $8.00 / g | 1KG | 99% | 5ton/Month | Qiuxian Baitai New Material Co., LTD |
-

- Dolutegravir
1051375-16-6
- US $120.00-80.00 / g
- 98%HPLC
- Shenzhen Nexconn Pharmatechs Ltd
-

- Dolutegravir; DTG; GSK1349572
1051375-16-6
- US $240.00 / g
- 99% Purity (What/sapp: +86 18145728414)
- Guangzhou Tengyue Chemical Co., Ltd.
-

- GSK1349572
1051375-16-6
- US $8.00 / g
- 99%
- Qiuxian Baitai New Material Co., LTD