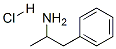
D-AMPHETAMINE HYDROCHLORIDE
- CAS No.
- 51-64-9
- Chemical Name:
- D-AMPHETAMINE HYDROCHLORIDE
- Synonyms
- Dexamfetamine;D-AMPHETAMINE;Dexedrine;(S)-1-phenylpropan-2-amine;Dexamphetamine;C07884;Dexacaps;dexidrine;NSC-73713;amphetamine(d)
- CBNumber:
- CB6266202
- Molecular Formula:
- C9H13N
- Molecular Weight:
- 135.21
- MDL Number:
- MFCD01708025
- MOL File:
- 51-64-9.mol
| Melting point | 25°C |
|---|---|
| Boiling point | 208.93°C (estimate) |
| Density | 0.9354 (estimate) |
| refractive index | 1.5087 (estimate) |
| Flash point | 9℃ |
| storage temp. | -20°C |
| pka | pKa 9.90 (Uncertain) |
| color | Oil |
| FDA UNII | TZ47U051FI |
| NCI Drug Dictionary | Dexedrine |
| ATC code | N06BA02 |
| EPA Substance Registry System | Dextroamphetamine (51-64-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H225-H301+H311+H331-H370 |
| Precautionary statements | P210-P260-P280-P301+P310-P311 |
| Hazard Codes | F,T |
| Risk Statements | 11-23/24/25-39/23/24/25 |
| Safety Statements | 16-36/37-45 |
| RIDADR | 3249 |
| WGK Germany | 1 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| Toxicity | LD50 orl-rat: 38 mg/kg TXAPA9 18,185,71 |
D-AMPHETAMINE HYDROCHLORIDE Chemical Properties,Uses,Production
Uses
CNS stimulant; anorexic. More active isomer of Amphetamine. Induces release of catecholamines and serotonin by displacing the monoamines from their vesicular storage sites; blocks catecholamine reuptake. Controlled substance (stimulant)
Definition
ChEBI: A 1-phenylpropan-2-amine that has S configuration.
brand name
Dexampex (Teva); Dexedrine (GlaxoSmithKline); Dextrostat (Shire); Ferndex (Ferndale);Adiparthrol;Afatin;Amfe-dyn;Amphaetex;Bipheramine;Curban;D-amfetasul;Dexadrine;Dexamed;Dexedrina;Dexten;Dextro-profetamine;Drinamyl;Durophet-m;Maxiton;Mephadexamine-r;Mephadexamin-r;Obetrol;Obotan;Proptan;Robese;Simpamina d;Steladex;Stil-2;Synatan.
World Health Organization (WHO)
Schedule II of the 1971 Convention on Psychotropic Substances. See WHO comment for amfetamine. (Reference: (UNCPS2) United Nations Convention on Psychotropic Substances (II), , , 1971)
Safety Profile
Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Experimental reproductive effects. Chronic exposure causes central nervous system damage and blood-pressure effects. When heated to decomposition it emits toxic NOx. See other amphetamine entries.
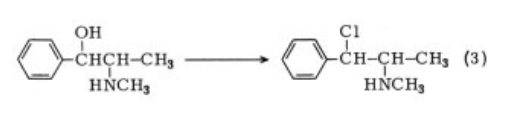
Synthesis
The compound can be prepared by optical
resolution of amphetamineor directly
from (-)-norephedrine by conversion to
the (+)-chloroamine followed by catalytic hydrogenation .