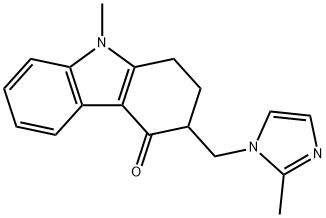
Ondansetron
- CAS No.
- 99614-02-5
- Chemical Name:
- Ondansetron
- Synonyms
- ONDANSETRON HCL;ONDANSETRON HYDROCHLORIDE DIHYDRATE;ONDANSETRON HCL DIHYDRATE;Ondanestron;Yatrox;gr38032;gr38032x;Ondanles;GR 38032F;HSDB 6544
- CBNumber:
- CB6340203
- Molecular Formula:
- C18H19N3O
- Molecular Weight:
- 293.36
- MDL Number:
- MFCD00764297
- MOL File:
- 99614-02-5.mol
| Melting point | 231-232° |
|---|---|
| Boiling point | 435.21°C (rough estimate) |
| Density | 1.1385 (rough estimate) |
| refractive index | 1.5855 (estimate) |
| storage temp. | −20°C |
| solubility | H2O: >5 mg/mL |
| form | solid |
| pka | pKa 7.4 (Uncertain) |
| color | white |
| CAS DataBase Reference | 99614-02-5(CAS DataBase Reference) |
| FDA UNII | 4AF302ESOS |
| NCI Drug Dictionary | ondansetron |
| ATC code | A04AA01 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H318-H410 | |||||||||
| Precautionary statements | P264-P273-P280-P301+P310+P330-P305+P351+P338+P310-P391 | |||||||||
| Hazard Codes | T,Xi | |||||||||
| Risk Statements | 25-36/37/38 | |||||||||
| Safety Statements | 45-37/39-26 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FE6375500 | |||||||||
| HS Code | 29339900 | |||||||||
| Toxicity | TDLo vn-man: 229 mg/kg/I LANCAO 344,190,94 | |||||||||
| NFPA 704 |
|
Ondansetron price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1478571 | Ondansetron | 99614-02-5 | 300mg | $581 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP806 | Ondansetron impurity standard British Pharmacopoeia (BP) Reference Standard | 99614-02-5 | 25MG | $236 | 2023-06-20 | Buy |
| Cayman Chemical | 21710 | Ondansetron (hydrochloride) (CRM) | 99614-02-5 | 1mg | $117 | 2024-03-01 | Buy |
| TRC | O655005 | Ondansetron | 99614-02-5 | 500mg | $835 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0031669 | ONDANSETRON-D5 95.00% | 99614-02-5 | 10MG | $903.5 | 2021-12-16 | Buy |
Ondansetron Chemical Properties,Uses,Production
Abstract
Ondansetron is used to prevent nausea and vomiting that may be caused by surgery, cancer chemotherapy, or radiation treatment. The 5-HT3 receptor antagonists in Ondansetron are the primary drugs used to treat and prevent chemotherapy-induced nausea and vomiting and radiotherapy-induced nausea and vomiting, through blocking the actions of chemicals in the body.
The efficacy is better than metoclopramide while less sedating than cyclizine or droperidol. However, it has little effect on vomiting caused by motion sickness. It can be given by mouth, by injection into a muscle or into a vein.
Mechanism of action
Ondansetron and granisetron, dolasetron are three common clinically used antiemetics, ondansetron is an effective serotonin (5-HT3) receptor blocker which is reversible and selective, for α1, α2, β1, β2-adrenergic receptors and the histamine H1, H2 receptors ,it has the minimal effect ,for H receptors, central and peripheral dopaminergic receptors ,it has no antagonistic effect ,it can suppress the chemotherapy and radiotherapy-induced nausea and vomiting. Compared with metoclopramide, its antiemetic effect is stronger and it has no extrapyramidal reactions. For vomiting induced by cisplatin, cyclophosphamide, doxorubicin, etc. it can produce rapid and strong antiemetic effect. It is suitable not only for the treatment of nausea and vomiting caused by the cytotoxic chemotherapy and radiation therapy, but also for the prevention and treatment of nausea and vomiting induced by surgeries.
Ondansetron works as a transit point between the visceral afferent nerve activated in the gastrointestinal tract and vomiting center within the spinal cord , which leads to the diaphragm and abdominal muscles movements. Chemotherapy and radiation therapy can cause intestinal 5-HT release and cause vagus nerve stimulation by 5-HT3 receptor ,which causes vomiting reflex. This product blocks this reflex occurring ,at the same time it blocks the vomiting triggered by the central action. The mechanism about postoperative nausea and vomiting is unknown. Ondansetron in combination with dexamethasonecan can enhance the anti-emetic effect.
Chemical properties
It is crystallized from methanol, mp 231-232 ℃.
Ondansetron hydrochloride dihydrate : C18H19N3O·ClH·2H2O[99614-01-4]. From the water-isopropanol , white crystalline solid is generated, mp 178.5~179.5 ℃.
Ondansetron Hydrochloride Monohydrate: C18H19N3O·HCl·H2O. Crystallization, melting point 186~187 ℃.
3S-type: [α] D25-14 ° (C = 0.19, methanol).
3R-type: [α] D24 + 16 ° (C = 0.34, methanol).
Application
As serotonin (5-HT3) receptor antagonists, it belongs to efficient antiemetic drugs,it has high strength and high selectivity, and it can control the receptor stimulation caused vomiting in the small intestine and CTZ (chemoreceptor trigger zone).It is used for treatment of chemotherapy and radiotherapy-induced emesis ,in particular ,it is used for the treatments of the emetic effect caused by cisplatin, dacarbazine, mechlorethamine, doxorubicin . It has no extrapyramidal effects ,and it can be used in conjunction with acetylcholine,its antiemetic effect is better than Metoclopramiden, Dompesidone, phenothiazines and butyrophenones.
Preparation
First preparation method:
After the reaction of 2-bromo-aniline and 1,3-cyclohexanedione,use dehydrobromination cyclization to produce the tetrahydrocarbazole derivative, and then react with polyformaldehyde and dimethylamine, then introduce dimethylaminomethyl in position 2 , the compound (ⅲ) is obtained. 3.80g compound (Ⅲ) reacts with methyl iodide, methyl group is introduced at position 9 while the side-chain amino is quaternized ,to give 5.72g compound (Ⅳ). 2.0g compound (Ⅳ) and 2-methyl-1H-imidazole in dimethyl formamide, stir and react at 95 ℃, to give 0.60g ondansetron.
Second preparation method:
cyclohexanone reacts with phenylhydrazine to give tetrahydrocarbazole in 85% yield. This is dissolved in tetrahydrofuran and water, under nitrogen,it is added dropwise the tetrahydrofuran solution of 2,3,5,6-tetrachloro-1,4-benzoquinone at 0℃, stir to give the oxidation product (Ⅱ), in a yield of 67.4% .
Compound (Ⅱ), ethanol, concentrated hydrochloric acid, paraformaldehyde and dimethylamine hydrochloride,are refluxing together. After Process, then in acetone, concentrated hydrochloric acid is added ,then stir at 50℃ to give methylation product (the V), in a yield of 71.7%.
Compound (V) and 2-methylimidazole in water,react at 110 ℃ reaction, the compound (Ⅵ)is generated, in a yield of 70.9%.
Compound (Ⅵ), methyl iodide and potassium carbonate, are stirred at room temperature until solid disappears . Pour it into water, stir , filter , wash with water and recrystallize from methanol to give ondansetron,in a yield of 57.2%. It is dissolved in a mixture of acetone and water, concentrated hydrochloric acid is added ,after the reaction , ondansetron hydrochloride dihydrate can be obtained , the yield is 92.6%
Third preparation method:
Compound (II), potassium carbonate, acetone and dimethyl sulfate, are stirred at room temperature. Compound (Ⅶ)is generated , the yield is 91%.
Compound (Ⅶ) is dissolved in ethanol ,at reflux ,there is the batch addition of a mixture of paraformaldehyde and dimethylamine hydrochloride.After the addition is finished, reflux. After treatment, the compound (Ⅷ)is obtained, the yield is 67%.
(Ⅷ) is dissolved in ethanol, inlet hydrogen chloride gas, obtaining its hydrochloride. The hydrochloride is added to water, 2-Methylimidazole is added at 50 ℃ , reflux to obtain ondansetron,the yield is 70%. This is dissolved in isopropyl alcohol, water and concentrated hydrochloric acid, and it is stirred at room temperature to obtain ondansetron hydrochloride dihydrate, the yield is 90.5%.
References
https://en.wikipedia.org
https://www.drugs.com/ondansetron.html
Description
Ondansetron (hydrochloride) (CRM) (Item No. 21710) is a certified reference material categorized as an antiemetic. Formulations containing ondansetron have been used to reduce alcohol consumption and mood disturbances in early-onset alcoholics. This product is intended for research and forensic applications.
Chemical Properties
White to yellow crystal
Originator
Ondansetron hydrochloride ,Chemo Iberica , Spain
Uses
antibacterial
Indications
Ondansetron (Zofran) is potent antagonists of 5-HT3 receptors,which is found peripherally on vagal nerve terminals and centrally in the CTZ. During chemotherapy that induces vomiting, mucosal enterochromaffin cells in the GI tract release serotonin, which stimulates 5-HT3 receptors.
Definition
ChEBI: Ondansetron is a member of carbazoles.
Manufacturing Process
Preparation of 3-ethoxalyl-9-methyl-1,2,3,9-tetrahydro-4H-carbozol-4-one
3.0 g (0.13 mole) of sodium metal are portionwise added to a stirred mixture containing 19.93 g (0.1 mole) of 9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4one, 19.0 g (0.13 mole) of diethyl oxalate, 2 g of ethanol and 200 ml of dioxane. The slightly warming reaction mixture is stirred at 40° to 50°C for 4 hours, then 16 g of glacial acetic acid and finally 200 ml of water are added thereto at room temperature. After filtering off the yellow crystalline suspension, the precipitate is washed with water and dried to give the title compound in a yield of 24 g (80.2%), m.p. 118°-120°C
Preparation of 3-hydroxymethyl-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4one-3-glyoxylic acid lacton
After adding 0.1 g of triethylamine to a stirred suspension containing 3.00 g (0.01 mole) of the 3-ethoxalyl-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4
2512 one, in 20 ml of acetone, 1.13 g (0.015 mole) of formol solution are dropwise added to the mixture. The suspension becomes clear within 1 to 2 minutes and crystals begin to precipitate. After further stirring at 35° to 40°C for one hour, the reaction mixture is cooled down to room temperature, filtered off, the precipitate is washed with 50% acetone and dried to give 2.10 g (74.2%) of the title compound, m.p. 242°-244°C.
Preparation of ondansetron base (chemically 9-methyl-3-[(2-methyl-1-Himidazol-1-yl)methyl]-1,2,3,9-tetrahidro-4-H-carbazol-4-one)
A mixture containing 2.83 g (0.01 mole) of 3-hydroxymethyl-9-methyl-2,3,9tetrahydro-4H-carbazol-4-one-3-glyoxylic acid lactone, 15 ml of dioxane, 1.32 g of triethylamine, 1.0 g of ethanol and 1.64 g (0.02 mole) of 2methylimidazole is boiled under reflux while stirring for 5 hours. Thereafter, the reaction mixture is diluted with 45 ml of water and cooled down. The precipitate is filtered off, washed with aqueous dioxane and dried to obtain 2.56 g (87.3%) of the title compound, m.p. 220°-223°C.
Preparation of 9-methyl-3-[(2-methyl-1-H-imidazol-1-yl)methyl]-1,2,3,9tetrahydro-4H-carbazol-4-one hydrochloride dihydrate The process above described is followed, except that after cooling down the reaction mixture to room temperature after boiling, 20 ml of 37% aqueous hydrochloric acid are added thereto. Then, the precipitate is filtered off, washed with isopropanol and dried to obtain 2.40 g (65.6%) of the title salt, m.p. 178°-180°C. The active agent content of the product was found to be 100.3% based on potentiometric titration with sodium hydroxide solution. The theoretical water content is 9.85% (calculated for C18H19N3OHCl2H2O).The water content measured is 10.03%.
brand name
Zofran (GlaxoSmithKline).
Therapeutic Function
Serotonin antagonist
Clinical Use
Ondansetron
Side effects
This causes vagal afferent discharge, inducing vomiting. In binding to 5-HT3 receptors, ondansetron blocks serotonin stimulation, hence vomiting, after emetogenic stimuli such as cisplatin. Headache is the most frequently reported adverse effect of these medications.
Safety Profile
A poison by intravenous route.Human systemic effects by intravenous route: jaundice.When heated to decomposition it emits toxic vapors ofNOx.
Veterinary Drugs and Treatments
Used as an antiemetic when conventional antiemetics are ineffective, such as when administering cisplatin or for other causes of intractable vomiting. The use of ondansetron in cats is somewhat controversial and some state it should not be used in this species.
Drug interactions
Potentially hazardous interactions with other drugs
Cytotoxics: possible increased risk of ventricular
arrhythmias with panobinostat and vandetanib.
Dopaminergics: possible increased risk of
hypotension with apomorphine - avoid.
Metabolism
Ondansetron is metabolised in the liver through multiple enzymatic pathways; it is a substrate for cytochrome P450 isoenzymes, primarily CYP3A4, but also CYP1A2 and CYP2D6. The metabolites do not contribute to the pharmacological activity of ondansetron. Less than 5% of a dose is excreted unchanged in the urine
Ondansetron Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 | faith@widelychemical.com | CHINA | 742 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3759 | 58 |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | contact@fuxinpharm.com | China | 10297 | 58 |
View Lastest Price from Ondansetron manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-11-27 | Ondansetron
99614-02-5
|
US $735.00 / KG | 1KG | 99% min | 20 TONS | Wuhan Senwayer Century Chemical Co.,Ltd | |
 |
2022-10-10 | Ondansetron
99614-02-5
|
US $0.00-0.00 / kg | 1kg | 98% | 1Ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2021-08-05 | Ondansetron USP/EP/BP
99614-02-5
|
US $1.10 / g | 1g | 0.999 | 100 Tons min | Dideu Industries Group Limited |
-

- Ondansetron
99614-02-5
- US $735.00 / KG
- 99% min
- Wuhan Senwayer Century Chemical Co.,Ltd
-

- Ondansetron
99614-02-5
- US $0.00-0.00 / kg
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- Ondansetron USP/EP/BP
99614-02-5
- US $1.10 / g
- 0.999
- Dideu Industries Group Limited