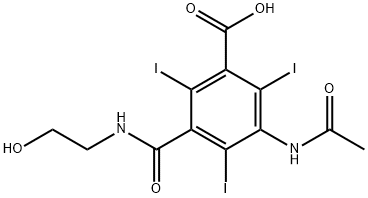
IOXITALAMIC ACID
- CAS No.
- 28179-44-4
- Chemical Name:
- IOXITALAMIC ACID
- Synonyms
- AG-58107;Telebrix;Vasobrix;Ioxitalamic;IoxitalaMate;IOXITALAMIC ACID;iooxitalamic acid;Acidum Joxitalamicum;IOXITALAMIC ACID USP/EP/BP;3-Acetamido-5-(2-hydroxyethylcarbamoyl)-2,4,6-triiodobenzoic acid
- CBNumber:
- CB6406686
- Molecular Formula:
- C12H11I3N2O5
- Molecular Weight:
- 643.94
- MDL Number:
- MFCD00867942
- MOL File:
- 28179-44-4.mol
| Melting point | 253-255?C |
|---|---|
| Boiling point | 582.8±50.0 °C(Predicted) |
| Density | 2.519±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| form | Solid |
| pka | 0.85±0.10(Predicted) |
| color | Off-White to Pale Brown |
| FDA UNII | 967RDI7Z6K |
| ATC code | V08AA05 |
IOXITALAMIC ACID price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | I738100 | IoxitalamicAcid | 28179-44-4 | 1g | $1065 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0069200 | IOXITALAMIC ACID 95.00% | 28179-44-4 | 10MG | $2009.7 | 2021-12-16 | Buy |
| Matrix Scientific | 099667 | 3-Acetamido-5-((2-hydroxyethyl)carbamoyl)-2,4,6-triiodobenzoic acid 95+% | 28179-44-4 | 1g | $775 | 2021-12-16 | Buy |
| Matrix Scientific | 099667 | 3-Acetamido-5-((2-hydroxyethyl)carbamoyl)-2,4,6-triiodobenzoic acid 95+% | 28179-44-4 | 250mg | $327 | 2021-12-16 | Buy |
| AK Scientific | Z3684 | Ioxitalamicacid | 28179-44-4 | 250mg | $491 | 2021-12-16 | Buy |
IOXITALAMIC ACID Chemical Properties,Uses,Production
Chemical Properties
White Solid
Originator
Oxilan,Cook Imaging Corporation
Uses
A substituted 2,4,6-triiodobenzoic acid, an excellent contrast media for ventriculography, radiculography, lumbar myelography and x-rays of the cardiovascular system.
Definition
ChEBI: An organoiodine compound that is 2,4,6-triiodobenzoic acid substituted by an acetylamino group at position 3 and a (2-hydroxyethyl)carbamoyl group at position 5. It is used as a contrast medium.
Manufacturing Process
3-Methoxycarboxyl-5-nitrobenzoic acid (25 g) was hydrogenated in methanol
(500 ml) using palladium oxide on charcoal (2.5 g 10%) at atmospheric
pressure. When the exothermic reaction was completed the catalyst was
fluttered off. After cooling the solution at -20°C for 2.5 h, 12.7 g of 3-amino-
5-methoxycarbonylbenzoic acid was isolated. An additional 6.5 g of it was
isolated by concentrating the mother liquor.
The 3-amino-5-methoxycarbonylbenzoic acid (12.0 g) was suspended in water
(280 ml), dissolved by addition of concentrated hydrochloric acid (7.1 ml) and
glacial acetic acid (28.5 ml). At 60°-70°C NaICl2 solution (73 ml, 58.7 g
ICl/100 ml) was added dropwise while stirring in the course of about 3 h. The
reaction mixture was heated at 80°-90°C for additional 3 h while stirring.
After cooling to room temperature the mother liquor was decanted and the
residue dissolved as ammonium salt in water (80 ml). The ammonium salt
was precipitated by adding ammonium chloride (2.4 g) and cooling to 0°C.
The ammonium salt was filtered off and dissolved in water (140 ml),
charcoaled twice at 80°C and the acid was precipitated at room temperature
by addition of hydrochloric acid and was filtered off. The crude product was
dissolved in ethyl acetate (100 ml) and the solution was washed 3 times with
hydrochloric acid (2 N). By evaporating the solvent, 19 g of 3-amino-5-
methoxycarbonyl-2,4,6-triiodobenzoic acid was isolated. Melting point 170°-
176°C.
A mixture of 3-amino-5-methoxycarbonyl-2,4,6-triiodobenzoic acid (198 g)
and thionyl chloride (400 ml) was heated while stirring at 70°C for 16 h. The
solid material dissolved slowly. Thionyl chloride was evaporated in vacuo, the
residue dissolved in chloroform (1000 ml), the solution washed with water (80
ml each), twice with saturated sodium bicarbonate, then 5 times with 2 N
sodium hydroxide solution and finally with water to neutral. The solution was
dried with CaCl2 filtered and evaporated to dryness. The 3-amino-5-
methoxycarbonyl-2,4,6-triiodobenzoyl chloride was dried at 50°C in vacuo.
Yield: 203.0 g. Melting point 55°-60°C.
To the 3-amino-5-methoxycarbonyl-2,4,6-triiodobenzoyl chloride (53.0 g) was
added acetic anhydride (106 ml). After stirring at room temperature for 20
min then insoluble material was filtered off (3-4 g). To the filtrate was added
concentrated sulfuric acid (0.3 ml) whereby a yellowish product started to
precipitate. The temperature reached about 50°C. The 3-acetamido-5-
methoxycarbonyl-2,4,6-triiodobenzoyl chloride was isolated after storing in
refrigerator overnight. Yield: 39.0 g. Melting point 210°-215°C.
The 3-acetamido-5-methoxycarbonyl-2,4,6-triiodobenzoyl chloride was
dissolved in a mixture of dioxan and dimethylformamide. In the course of 2 h
this solution was added dropwise to a solution of ethanolamine and
triethylamine in dioxan. The stirring was continued. A sticky precipitate was
filtered off. The filtrate was evaporated to dryness in vacuo. The residue was
triturated with aqueous sodium bicarbonate, filtered off and mixed with first
fraction. The combined solids were then suspended in aqueous sodium
bicarbonate filtered off washed with water and dried in vacuo to give methyl
5-acetamido-2,4,6-triiodo-(N-β-hydroxyethyl)-isophthalamate.
The methyl 5-acetamido-2,4,6-triiodo-(N-β-hydroxyethyl)-isophthalamate was
mixed with fresh distilled ethanolamine and stirred. The excess ethanolamine
was removed in vacuo at 50°-60°C. The residue was dissolved in water, and
charcoaled at pH 5.5. The crude product was precipitated with hydrochloric
acid (pH 0.5) and filtered after stirring at 0°C. 5-Acetamido-2,4,6-triiodo-(N-
β-hydroxyethyl)isophthalamic acid was suspended in ethanol and dissolved by
addition of concentrated ammonia. The ammonium salt started to precipitate
in the course and was isolated after stirring. The salt was dissolved in water,
filtered and the acid was precipitated with hydrochloric acid (pH 0.5). After
stirring the product was filtered off and dried in vacuo.
Therapeutic Function
Diagnostic aid
IOXITALAMIC ACID Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
IOXITALAMIC ACID Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9409 | 58 |
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 | erin@hbldbiotech.com | China | 878 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29322 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
View Lastest Price from IOXITALAMIC ACID manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-10-20 | IoxitalaMic Acid
28179-44-4
|
US $350.00 / KG | 1KG | 99% | 9000kg/per week | Hebei Lingding Biotechnology Co., Ltd. | |
 |
2021-08-14 | IOXITALAMIC ACID USP/EP/BP
28179-44-4
|
US $1.10 / g | 1g | 0.999 | 100 Tons min | Dideu Industries Group Limited | |
 |
2020-01-05 | IOXITALAMIC ACID
28179-44-4
|
US $1.00 / KG | 1KG | 95--99% | 1ton | Career Henan Chemical Co |
-

- IoxitalaMic Acid
28179-44-4
- US $350.00 / KG
- 99%
- Hebei Lingding Biotechnology Co., Ltd.
-

- IOXITALAMIC ACID USP/EP/BP
28179-44-4
- US $1.10 / g
- 0.999
- Dideu Industries Group Limited
-

- IOXITALAMIC ACID
28179-44-4
- US $1.00 / KG
- 95--99%
- Career Henan Chemical Co
28179-44-4(IOXITALAMIC ACID)Related Search:
1of4