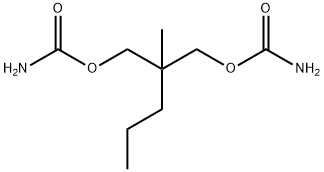
Meprobamate
- CAS No.
- 57-53-4
- Chemical Name:
- Meprobamate
- Synonyms
- Erina;Dapaz;Diron;Klort;Letyl;CaMHA;Oasil;paxin;Seril;Urbil
- CBNumber:
- CB6477952
- Molecular Formula:
- C9H18N2O4
- Molecular Weight:
- 218.25
- MDL Number:
- MFCD00063428
- MOL File:
- 57-53-4.mol
| Melting point | 97-100°C |
|---|---|
| Boiling point | 358.93°C (rough estimate) |
| Density | 1.2004 (rough estimate) |
| refractive index | 1.4480 (estimate) |
| Flash point | 11 °C |
| storage temp. | 2-8°C |
| solubility | DMF: 50 mg/ml; DMF:PBS(pH 7.2)(1:1): 0.5 mg/ml; DMSO: 30 mg/ml; Ethanol: 25 mg/ml |
| pka | 13.09±0.50(Predicted) |
| Water Solubility | 6.2g/L(25 ºC) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 57-53-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 9I7LNY769Q |
| Proposition 65 List | Meprobamate |
| NCI Drug Dictionary | Mepron |
| ATC code | N05BC01,N05BC51,N05CX01 |
| NIST Chemistry Reference | 1,3-Propanediol, 2-methyl-2-propyl-, dicarbamate(57-53-4) |
| EPA Substance Registry System | Meprobamate (57-53-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 22-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 7-16-36/37-45 | |||||||||
| RIDADR | UN 1230 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TZ0175000 | |||||||||
| HS Code | 2924110000 | |||||||||
| Toxicity | LD50 i.p. in mice: 800 mg/kg (Berger) | |||||||||
| NFPA 704 |
|
Meprobamate Chemical Properties,Uses,Production
Chemical Properties
white crystalline powder
Originator
Equanil,Wyeth,US,1955
Uses
An anxiolytic. This is a controlled substance.
Definition
ChEBI: Meprobamate is an organic molecular entity.
Manufacturing Process
A solution containing 52.8 parts of 2-methyl-2-n-propyl-1,3-propanediol and 128 parts of acetone is added with stirring to 112 parts of liquid phosgene at such a rate that the temperature of the reaction is maintained at -5° to 0°C. The reaction is stirred one hour at about 0°C then cooled to -15°C. A cooled 30% solution of 32 parts of sodium hydroxide is added with stirring to the reaction at such a rate that the temperature is maintained at -15° to -5°C. The mixture is stirred for an additional ? hour at about 0°C then cooled to - 20°C. 180 parts of cooled ammonium hydroxide solution (28.6% NH3) are added while cooling and with stirring at such a rate that the temperature rises slowly to 20°C and stirring is continued for an additional ? hour. The mixture is poured with agitation into 1,700 parts of ice water. The solid which separates is removed by filtration and dried. Recrystallization from water gives 55 parts (63% of theoretical yield) of 2-methyl-2-n-propyl-1,3-propanediol dicarbamate, MP 104° to 105°C.
brand name
Amosene (Ferndale); Bamate (Alra); Equanil (Wyeth); Mepriam (Teva); Meprospan (Medpointe); Miltown (Medpointe); Neuramate (Halsey); Tranmep (Solvay Pharmaceuticals);3p bamte;Apo-meprobamate;Carb-a-med;Clindoorm;Cusitan;Daritran;Detensitral;Dolovisano;Dormilfo n;Dystoid;Edental;Equiner;Equqtrqte;Gene-bamate;Idemin;Indemin;Irs 109 a;Iterco;Juvamidon;Lan-dol;Lenicor;M.a.s.;M.p. trantabs;Margaris;Meditran;Mep-e;Meprobadal;Meprobil;Meprogesic q;Meprolin;Mepronel;Mepro-secergan 400;Meproserpina;Meprospan 400;Meproten;Meproyrin;Meprozine;Meriprobate;Microbamat;Midixin;Milspan;Miltown s-r;Neo-nervostal;Neo-tran;Neurocalm;Novomato;Novomepro;Nyktogen;Oasil procalmadiol;Odsil 10;Panquil;Pensive;Pentaneural;Pm 2;Pmb 4000;Prequil;Probal;Probasan;Probromato;Psico-retard;Quietidon;Regium;Seda baxacor;Sedavier;Selene;Selodorm;Serenade;Sintown;Sopanil;Spasmobamat;Tcm 200;Tcm 400;Trankilin;Trelmar;Tri-reumo-campil;Vasocalm;Vistabamate;Wescomep.
Therapeutic Function
Tranquilizer
World Health Organization (WHO)
Meprobamate, a bis-carbamate ester, was introduced in 1955 for the treatment of anxiety and was subsequently used as a sedative-hypnotic drug. Psychological and physical dependence can occur and abuse has been reported. Meprobamate is controlled under Schedule IV of the 1971 Convention on Psychotropic Substances. (Reference: (UNCPS4) United Nations Convention on Psychotropic Substances (IV), , , 1971)
General Description
Odorless white crystalline powder. Bitter taste. Solutions in water are neutral or slightly acidic.
General Description
Meprobamate,2-methyl-2-propyltrimethylene dicarbamate, 2-methyl-2-propyl-1,3-propanediol dicarbamate (Equanil, Miltown), is officiallyindicated as an antianxiety agent. It is also a sedative–hypnotic agent. It has several overall pharmacological properties resembling those of benzodiazepines and barbiturates.The mechanism of action underlying anxiolytic effectsis unknown but may involve effects on conductivity inspecific brain areas.It does not appear to act through effectson GABAergic systems.The drug is effective againstabsence seizures and may worsen generalized tonic–clonicseizures.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Meprobamate is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates.
Hazard
Central nervous system depressant, use restricted by law.
Fire Hazard
Flash point data for Meprobamate are not available; however, Meprobamate is probably combustible.
Clinical Use
Meprobamate is also a centrally acting skeletal musclerelaxant. The agents in this group find use in several conditions,such as strains and sprains that may produce acutemuscle spasm. They have interneuronal blocking propertiesat the level of the spinal cord, which are said to bepartly responsible for skeletal muscle relaxation.Also,the general CNS depressant properties they possess maycontribute to, or be mainly responsible for, the skeletalmuscle relaxant activity.
Safety Profile
Human poison by unspecified routes. Moderately toxic to humans and experimentally by ingestion. Experimental poison by intravenous, intraperitoneal, and subcutaneous routes. An experimental teratogen. Human systemic effects by ingestion: coma, blood pressure decrease, regional or general arteriolar constriction, dyspnea, cyanosis, respiratory depression, nausea or vomiting, and allergic skin dermatitis. Experimental reproductive effects. Mutation data reported. Implicated in aplastic anemia. Used as a tranquilizer. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Crystallise it from hot water, aqueous EtOH (m 104-105.5o) or xylene (m 104.1-105.3o). It can be an addictive drug. [Beilstein 3 IV 73.]
Meprobamate Preparation Products And Raw materials
Raw materials
1of2