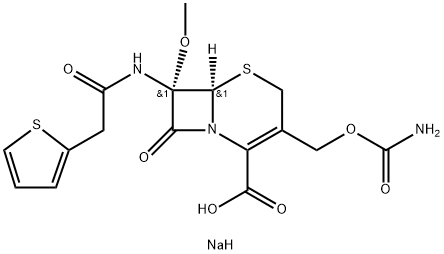
Cefoxitin sodium
- CAS No.
- 33564-30-6
- Chemical Name:
- Cefoxitin sodium
- Synonyms
- MK-306;MERXIN;BETACEF;FARMOXIN;(6R,7S)-;CENOMYCIN;MEFOXITIN;Mefoxithin;CEFOXITIN NA;cefoxotinsodium
- CBNumber:
- CB6495398
- Molecular Formula:
- C16H18N3NaO7S2
- Molecular Weight:
- 451.44
- MDL Number:
- MFCD00079042
- MOL File:
- 33564-30-6.mol
- MSDS File:
- SDS
| Melting point | >160°C |
|---|---|
| Boiling point | 843℃ |
| alpha | 25589nm +210° (c = 1 in methanol) |
| Flash point | >110°(230°F) |
| storage temp. | 2-8°C |
| solubility | Very soluble in water, sparingly soluble in alcohol. |
| color | White |
| Water Solubility | Soluble in water or methanol |
| InChIKey | GNWUOVJNSFPWDD-XMZRARIVSA-M |
| SMILES | N([C@@]1(C(=O)N2C(=C(COC(=O)N)CS[C@]12[H])C(=O)O)OC)C(=O)CC1SC=CC=1.[NaH] |&1:1,14,r| |
| CAS DataBase Reference | 33564-30-6(CAS DataBase Reference) |
| FDA UNII | Q68050H03T |
| NCI Drug Dictionary | cefoxitin sodium |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317 |
| Precautionary statements | P280-P302+P352 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 36/37/38-42/43 |
| Safety Statements | 22-26-36/37-36/37/39 |
| RIDADR | 3077 |
| WGK Germany | 3 |
| RTECS | XI0330500 |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 29419000 |
| Toxicity | LD50 in mice, rats, dogs (g/kg): 5.10, 8.98, >10.0 i.v. (Takayama) |
Cefoxitin sodium price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C4786 | Cefoxitin sodium salt analytical standard | 33564-30-6 | 1g | $372 | 2024-03-01 | Buy |
| Sigma-Aldrich | C4786 | Cefoxitin sodium salt analytical standard | 33564-30-6 | 5g | $878 | 2024-03-01 | Buy |
| TCI Chemical | C3602 | Cefoxitin Sodium | 33564-30-6 | 1G | $135 | 2024-03-01 | Buy |
| TCI Chemical | C3602 | Cefoxitin Sodium | 33564-30-6 | 5G | $420 | 2021-12-16 | Buy |
| Alfa Aesar | J66800 | Cefoxitin sodium, 92.7-97% | 33564-30-6 | 1g | $189 | 2024-03-01 | Buy |
Cefoxitin sodium Chemical Properties,Uses,Production
Brand Name(s) in US
Mefoxin
Description
Cefoxitin is a second generation cephalosporin antibiotic that has been used to treat a wide range of gram-
Chemical Properties
Solid
Originator
Mefoxin,Merck Sharp and Dohme,US,1978
Uses
A broad spectrum antibiotic
Uses
An antibiotic derived from Cephamycin C.
Uses
Cefoxitin sodium can be used as semi-synthetic antibiotic derived from Cephamycin C, possessing high resistance to β-lactamase inactivation.
Definition
ChEBI: Cefoxitin sodium is an organic molecular entity.
Manufacturing Process
Benzhydryl 3-carbamoyloxymethyl-7α-hydroxy-7β-(2-thienylacetamido)
decephalosporanate, 543 mg, is stirred in 15 ml dry DMSO. Sodium hydride,
24 mg (48 mg of a 50% suspension of NaH in mineral oi1, which has been
washed with hexane to remove the oil), is added. When hydrogen evolution
has ceased, 126 mg dimethyl sulfate is added. The solution is stirred for one
hour at room temperature, diluted with 100 ml benzene and washed six times
with water; the last wash is made to pH 8, if necessary, by adding sodium
bicarbonate. The solution is dried over MgSO4, filtered and evaporated,
leaving benzhydryl 3-carbamoyloxymethyl-7β-(2-thienylacetamido)-7α-
methoxydecephalosporanate, which may be purified if desired by
chromatography on silica gel, eluting with 25:1 chloroformethyl acetate.
Other methylating agents may be used in place of methyl sulfate, e.g., an
equimolar amount of methyl iodide, bromide or chloride, using the same
conditions, or methyl trifluoromethylsulfonate or trimethyloxonium
trinitrobenzenesulfonate. The solvent in the latter two reagents is dimethyl
ether-HMPA 1:1, using a reaction temperature of -20°C warming later to
25°C. In each instance, the benzhydryl 3-carbamoyloxymethyl-7β-(2-
thienylacetamido)-7α-methoxydecephalosporanate is obtained.
Benzhydryl 3-carbamoyloxymethyl-7β-(2-thienylacetamido)-7α-
methoxydecephalosporanate (300 mg) in 0.5 ml in anisole and 2.5 ml of
trifluoroacetic acid is reacted for 15 minutes at 10°C. The resulting mixture is evaporated at reduced pressure and flushed twice with anisole. The residue is
dissolved in methylene chloride and extracted with 5% sodium bicarbonate
solution. The aqueous solution is adjusted to pH 1.8 with 5% phosphoric acid
and extracted with ethyl acetate. The organic solution is dried and evaporated
to yield the pure 3-carbamoyloxymethyl-7α-methoxy-7β-(2-
thienylacetamido)decephalosporanic acid, MP 165°C to 167°C. This may then
be converted to the sodium salt.
brand name
Mefoxin (Merck).
Therapeutic Function
Antibiotic
Clinical Use
Cefoxitin (Mefoxin) is a semisynthetic derivative obtainedby modification of cephamycin C, a 7α-methoxy-substitutedcephalosporin isolated independently from variousStreptomyces by research groups in Japan and the UnitedStates. Although it is less potent than cephalothin againstGram-positive bacteria and cefamandole against most of theEnterobacteriaceae, cefoxitin is effective against certainstrains of Gram-negative bacilli (e.g., E. coli, K. pneumoniae,Providencia spp., S. marcescens, indole-positiveProteus spp., and Bacteroides spp.) that are resistant to thesecephalosporins. It is also effective against penicillin-resistantS. aureus and N. gonorrhoeae. The activity of cefoxitin and cephamycins, in general,against resistant bacterial strains is because of their resistanceto hydrolysis by β-lactamases conferred by the 7α-methoxylsubstituent. Cefoxitin is a potent competitive inhibitor ofmany β-lactamases. It is also a potent inducer of chromosomallymediated β-lactamases. The temptation to exploit the β-lactamase–inhibiting properties of cefoxitin by combining itwith β-lactamase–labile β-lactam antibiotics should be temperedby the possibility of antagonism. In fact, cefoxitin antagonizesthe action of cefamandole against E. cloacae andthat of carbenicillin against P. aeruginosa. Cefoxitin aloneis essentially ineffective against these organisms.
The pharmacokinetic properties of cefoxitin resemblethose of cefamandole. Because its half-life is relativelyshort, cefoxitin must be administered 3 or 4 times daily.Solutions of the sodium salt intended for parenteral administrationare stable for 24 hours at room temperature and 1week if refrigerated. 7α-Methoxyl substitution stabilizes, tosome extent, the β-lactam to alkaline hydrolysis.The principal role of cefoxitin in therapy seems to be forthe treatment of certain anaerobic and mixed aerobic–anaerobicinfections. It is also used to treat gonorrhea caused byβ-lactamase–producing strains. It is classified as a secondgenerationagent because of its spectrum of activity.
Veterinary Drugs and Treatments
In the United States, there are no cefoxitin products approved for veterinary species, but it has been used clinically in several species when an injectable second generation cephalosporin may be indicated.
References
[1]. miller ak, celozzi e, kong y, et al. cefoxitin, a semisynthetic cephamycin antibiotic: in vivo evaluation. antimicrob agents chemother. 1974 jan;5(1):33-7.
[2]. jacoby ga. ampc beta-lactamases. clin microbiol rev.2009 jan;22(1):161-82.
Cefoxitin sodium Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shenzhen Excellent Biomedical Technology Co.,Ltd. | +86-0755-26050679 +86-15915472436 | sale@ex-biotech.cn | China | 1031 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 775 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Shaanxi Yikanglong Biotechnology Co., Ltd. | 17791478691 | yklbiotech@163.com | CHINA | 296 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 | faith@widelychemical.com | CHINA | 742 | 58 |
View Lastest Price from Cefoxitin sodium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-10 | Cefoxitin
33564-30-6
|
US $0.00-0.00 / mg | 10mg | 90%+ | 10g | Guangzhou PI PI BIOTECH INC | |
 |
2024-04-09 | Cefoxitin sodium
33564-30-6
|
US $0.00 / Kg | 1Kg | 99% | 10kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2023-04-12 | Cefoxitin sodium
33564-30-6
|
US $1.00 / KG | 1KG | 99.25% HPLC | 5000tons/year | Wuhan Dujiang Industrial Co., Ltd. |
-

- Cefoxitin
33564-30-6
- US $0.00-0.00 / mg
- 90%+
- Guangzhou PI PI BIOTECH INC
-

- Cefoxitin sodium
33564-30-6
- US $0.00 / Kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Cefoxitin sodium
33564-30-6
- US $1.00 / KG
- 99.25% HPLC
- Wuhan Dujiang Industrial Co., Ltd.