Dipyridamole
- CAS No.
- 58-32-2
- Chemical Name:
- Dipyridamole
- Synonyms
- DIPYRIDAMOL;Dipiridamol;dypyridamole;Anginal;persantin;Persantine;Dipyridamole (200 mg);ra8;RA 8;Natyl
- CBNumber:
- CB6731869
- Molecular Formula:
- C24H40N8O4
- Molecular Weight:
- 504.63
- MDL Number:
- MFCD00010555
- MOL File:
- 58-32-2.mol
- MSDS File:
- SDS
| Melting point | 165-166 °C (lit.) |
|---|---|
| Boiling point | 593.96°C (rough estimate) |
| Density | 1.2046 (rough estimate) |
| refractive index | 1.6910 (estimate) |
| storage temp. | room temp |
| solubility | DMSO: soluble |
| form | powder |
| pka | pKa 6.4 (Uncertain) |
| color | yellow |
| Water Solubility | Soluble in chloroform, methanol, dilute acids, ethanol. Slightly soluble in water. |
| Merck | 14,3346 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| FDA UNII | 64ALC7F90C |
| NCI Dictionary of Cancer Terms | dipyridamole |
| NCI Drug Dictionary | dipyridamole |
| ATC code | B01AC07 |
| NIST Chemistry Reference | Pyrimidine, 2,6-bis(diethanolamino)-4,8-dipiperidino-pyrimido-(5,4-d)-(58-32-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | KK7450000 | |||||||||
| HS Code | 29335990 | |||||||||
| Toxicity | LD50 in rats: 8.4 g/kg orally; 208 mg/kg i.v. (Takenaka) | |||||||||
| NFPA 704 |
|
Dipyridamole price More Price(54)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D9766 | Dipyridamole ≥98% (HPLC) | 58-32-2 | 1g | $35.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP131 | Dipyridamole British Pharmacopoeia (BP) Reference Standard | 58-32-2 | 100MG | $262 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP1102 | Dipyridamole impurity standard British Pharmacopoeia (BP) Reference Standard | 58-32-2 | 25MG | $238 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1220506 | Dipyridamole United States Pharmacopeia (USP) Reference Standard | 58-32-2 | 200mg | $242.4 | 2024-03-01 | Buy |
| TCI Chemical | D2274 | Dipyridamole | 58-32-2 | 5g | $47 | 2024-03-01 | Buy |
Dipyridamole Chemical Properties,Uses,Production
Description
Dipyridamole (58-32-2) is a phosphodiesterase inhibitor (IC50=0.37, 0.38, 0.45, 0.9 and 4.5 μM for PDE11, 6, 10, 5 and 8 respectively.1,2 Potent equilibrative nucleoside transporter 1 (ENT1) inhibitor Ki=8.2 nM vs. 144.8 nM for ENT1 and ENT2 respectively.3 Antiplatelet activity.4
Chemical Properties
Yellow Powder
Originator
Persantine,Boehringer Ingelheim,US,1961
Uses
A phosphodiester, cGMP, and non-specific nucleoside transport inhibitor
Uses
Selective inhibitor of phosphodiesterase V (PDE 5); potent coronary vasodilator drug; adenosine transport inhibitor; inhibitor of platelet aggregation
Uses
Dipyridamole prevents platelets sticking to the replacement heart valve and causing a blood clot on the valve. It is used to dilate blood vessels in people with peripheral arterial disease and coronary artery disease. This compound has been shown to suppress high glucose-induced osteopontin mRNA expression and protein secretion, as well as inhibit cAMP and cGMP hydrolysis. Research indicates that Dipyridamole is a non-specific nucleoside transport inhibitor with the ability to increase the effects of adenosine in sinoatrial and atrioventricular nodes. Dipyridamole is an inhibitor of ENT1 and ENT2.
Uses
Dipiridamol is known as a coronary vasodilating agent, although it also possesses specific antiaggregant activity. It is used for preventing thrombo-formation after cardiac valve replacement in combination with warfarin.
Definition
ChEBI: A pyrimidopyrimidine that is 2,2',2'',2'''-(pyrimido[5,4-d]pyrimidine-2,6-diyldinitrilo)tetraethanol substituted by piperidin-1-yl groups at positions 4 and 8 respectively. A vasodilator agent, it inhibits the formation of blood clots.
Manufacturing Process
Urea may be reacted with acetoacetic ester and that product nitrated to give 5-nitro-orotec acid. That is hydrogenated, then reacted with urea and potassium cyanate to give tetrahydroxypyrimidopyrimidine. The tetrahydroxy compound is converted to the tetrachloro compound POCl3. Reaction with diethanolamine and then with piperidine gives dipyridamole.
brand name
Persantine (Boehringer Ingelheim).
Therapeutic Function
Coronary vasodilator
General Description
Dipyridamole, 2,2',2',2'''-[(4,8-di-1piperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetrakisethanol (Persantine), may be used for coronary andmyocardial insufficiency. Its biggest use today, however, isas an antithrombotic in patients with prosthetic heart valves.It is a bitter, yellow, crystalline powder, soluble in diluteacids, methanol, or chloroform. A formulation containingdipyridamole and aspirin (Aggrenox) is currently beingmarketed as an antithromobotic.
Dipyridamole is a long-acting vasodilator. Its vasodilatingaction is selective for the coronary system; it is indicatedfor long-term therapy of chronic angina pectoris. Thedrug also inhibits adenosine deaminase in erythrocytesand interferes with the uptake of the vasodilator adenosineby erythrocytes. These actions potentiate the effect ofprostacyclin (PGI2), which acts as an inhibitor to plateletaggregation.
Biological Activity
Coronary vasodilator; adenosine transport inhibitor. Phosphodiesterase inhibitor (IC 50 values are 0.37, 0.38, 0.45, 0.9 and 4.5 μ M? for PDE11, 6, 10, 5 and 8 respectively).
Biochem/physiol Actions
Selective inhibitor of phosphodiesterase V (PDE 5); potent coronary vasodilator drug; adenosine transport inhibitor; inhibitor of platelet aggregation.
Mechanism of action
Dipyridamole exerts its antiplatelet function by increasing cellular concentrations of cAMP via its inhibition of the degradating enzyme, cyclic nucleotide PDE3. It also blocks adenosine uptake, which acts at A2 adenosine receptors to stimulate platelet adenyl cyclase. Less common uses for this drug include inhibition of embolization from prosthetic heart valves when used in combination with warfarin (the only currently recommended use) and reduction of thrombosis in patients with thrombotic disease when used in combination with aspirin. Alone, dipyridamole has little, if any, benefit in the treatment of thrombotic conditions.
Clinical Use
Dipyridamole is a pyrimidopyrimidine derivative with vasodilatory and antiplatelet properties.
Safety Profile
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion and subcutaneous routes. Human systemic effects cardiomyopathy including infarction. Mutation data reported. Used as a coronary vasodilator. When heated to decomposition it e
Synthesis
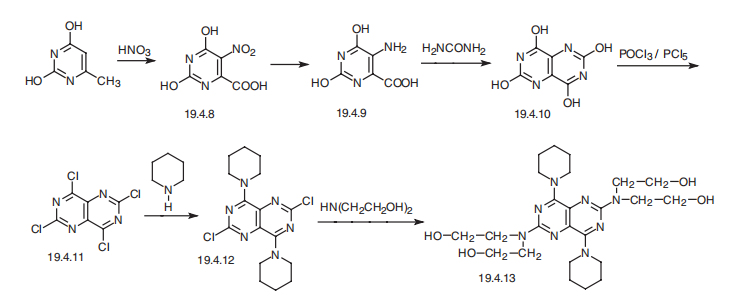
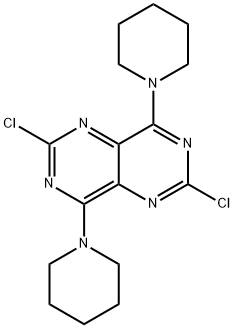
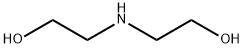
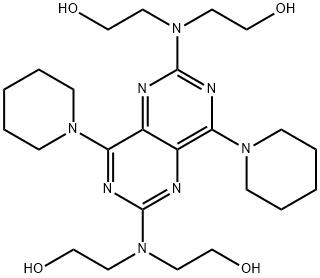
Dipyridamole, 2,2',2'',2'''-[(4,8-dipiperidinopirimido[5,4-d]pirimidin-2,6- diyl)-diimino]-tetraethanol (19.4.13), is easily synthesized from 5-nitroorotic acid (19.4.8), easily obtained, in turn, by nitrating of 2,4-dihydroxy-6-methylpyrimidine, which is usually synthesized by the condensation of urea with acetoacetic ether. Reduction of the nitro group in 5-nitroorotic acid by various reducing agents gives 5-aminoorotic acid (19.4.9), which is reacted with urea or with potassium cyanide to give 2,4,6,8- tetrahydroxypyrimido[5,4-d]pyrimidine (19.4.10). This undergoes a reaction with a mixture of phosphorous oxychloride and phosphorous pentachloride, which forms 2,4,6,8- tetrachloropyrimido[ 5,4-d]pyrimidine (19.4.11). Reacting the resulting tetrachloride with piperidine replaces the chlorine atoms at C4 and C8 of the heterocyclic system with piperidine, giving 2,6-dichloropyrimido-4,8-dipiperidino[5,4-d]pyrimidine (19.4.12). Reacting the resulting product with diethanolamine gives dipyridamole (19.4.13).
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: effects of adenosine enhanced and
extended.
Anticoagulants: anticoagulant effect of coumarins,
phenindione and heparin enhanced.
Metabolism
Dipyridamole is metabolised in the liver. Renal excretion of the parent compound is negligible (< 0.5%). Urinary excretion of the glucuronide metabolite is low (5%), the metabolites are mostly (about 95%) excreted via the bile into the faeces, with some evidence of entero-hepatic recirculation.
storage
Store at RT
References
1) Fujishige et al. (1999), Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A); J. Biol. Chem., 274 18438 2) Soderling et al. (1998), Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase; Proc. Natl. Acad. Sci. USA, 95 8991 3) Lin and Buolamwini (2007), Synthesis, flow cytometric evaluation, and identification of highly potent dipyridamole analogues as equilibrative nucleoside transporter 1 inhibitors.; J. Med. Chem., 50 3906 4) Coccheri (2010), Antiplatelet drugs – do we need new options? With a reappraisal of direct thromboxane inhibitors; Drugs, 70 887
Dipyridamole Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Huadong Medicine (Xi'an)Bodyguard Pharmaceutical Co.,Ltd. | +86-029-86185165 +8618629664246 | guoyuan@eastchinapharm.com | China | 1615 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Changzhou Rokechem Technology Co., Ltd. | 18758118018 | sales001@rokechem.com | China | 255 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | 2691956269@qq.com | China | 359 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | 18957127338 | stella@zetchem.com | China | 2141 | 58 |
View Lastest Price from Dipyridamole manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-19 | Dipyridamole
58-32-2
|
US $0.00-0.00 / KG | 1KG | 99 | 10 tons | Shanghai Affida new material science and technology center | |
 |
2024-03-16 | Dipyridamole
58-32-2
|
US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | |
 |
2023-08-18 | Dipyridamole
58-32-2
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- Dipyridamole
58-32-2
- US $0.00-0.00 / KG
- 99
- Shanghai Affida new material science and technology center
-

- Dipyridamole
58-32-2
- US $0.00 / KG
- 98%+
- WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD
-

- Dipyridamole
58-32-2
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd





