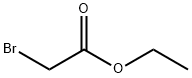
Ethyl bromoacetate
- CAS No.
- 105-36-2
- Chemical Name:
- Ethyl bromoacetate
- Synonyms
- ETHYL 2-BROMOACETATE;BROMOACETIC ACID ETHYL ESTER;Antol;ethylbromacetate;hyl bromoacetate;Ethyl-bromacetat;Ethyl bromacetate;ETHYL BROMOACETATE;ethylbromoethanoate;bromoacetated’ethyle
- CBNumber:
- CB6852654
- Molecular Formula:
- C4H7BrO2
- Molecular Weight:
- 167
- MDL Number:
- MFCD00000191
- MOL File:
- 105-36-2.mol
- MSDS File:
- SDS
| Melting point | -38 °C |
|---|---|
| Boiling point | 159 °C(lit.) |
| Density | 1.506 g/mL at 25 °C(lit.) |
| vapor density | 5.8 (vs air) |
| vapor pressure | 2.6 mm Hg ( 25 °C) |
| refractive index |
n |
| Flash point | 118 °F |
| storage temp. | Store below +30°C. |
| solubility | water: insoluble |
| form | Liquid |
| color | Clear |
| Specific Gravity | 1.506 |
| Odor | Pungent odour |
| Water Solubility | Miscible with ethanol, acetone, benzene and ethyl ether. Immiscible with water. |
| BRN | 506456 |
| Dielectric constant | 9.75 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, water, strong acids. |
| InChIKey | PQJJJMRNHATNKG-UHFFFAOYSA-N |
| CAS DataBase Reference | 105-36-2(CAS DataBase Reference) |
| EWG's Food Scores | 1-2 |
| FDA UNII | D20KFB313W |
| NIST Chemistry Reference | Acetic acid, bromo-, ethyl ester(105-36-2) |
| EPA Substance Registry System | Acetic acid, 2-bromo-, ethyl ester (105-36-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H300+H310+H330 | |||||||||
| Precautionary statements | P210-P233-P240-P280-P303+P361+P353-P304+P340+P310 | |||||||||
| Hazard Codes | T+ | |||||||||
| Risk Statements | 26/27/28 | |||||||||
| Safety Statements | 26-45-7/9-1/2 | |||||||||
| RIDADR | UN 1603 6.1/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | AF6000000 | |||||||||
| F | 19 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29159080 | |||||||||
| NFPA 704 |
|
Ethyl bromoacetate price More Price(25)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.01636 | Ethyl bromoacetate for synthesis | 105-36-2 | 250ML | $116 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.01636 | Ethyl bromoacetate for synthesis | 105-36-2 | 1L | $351 | 2024-03-01 | Buy |
| Sigma-Aldrich | 133973 | Ethyl bromoacetate reagent grade, 98% | 105-36-2 | 5g | $42.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 133973 | Ethyl bromoacetate reagent grade, 98% | 105-36-2 | 100g | $42.6 | 2024-03-01 | Buy |
| Alfa Aesar | A10448 | Ethyl bromoacetate, 98% | 105-36-2 | 100g | $39 | 2024-03-01 | Buy |
Ethyl bromoacetate Chemical Properties,Uses,Production
Description
Ethyl bromoacetate is a clear, colorless tolight-yellow liquid. Molecular weight = 167.02; Boilingpoint = 159℃. Freezing point ≤-20℃; Flashpoint = 48℃. Hazard Identification (based on NFPA-704 MRating System): Health 2, Flammability 2, Reactivity 0.Insoluble in water
Chemical Properties
Ethyl bromoacetate is a clear, colorless to light-yellow liquid and has a pungent odor. It is miscible with ethanol, ether, benzene and with other oxygenated and aromatic solvents, insoluble in water and partially decomposed by water.
Uses
Ethyl bromoacetate is widely used as an alkylating reagent involved in the Reformatsky reaction to prepare the beta-hydroxy esters by reacting with carbonyl compounds. It is also used for the syntheses of witting reagent, artificial diethylstilbestrol antigen, 3-phenyl-1-naphthol and steroidal thiazolidinone derivatives. It finds applications in the preparation of reversibly photoresponsive coumarin-stabilized polymeric nanoparticles in an aqueous medium which act as a detectable drug carrier.
Application
Ethyl Bromoacetate is used as a synthetic organic chemical intermediate as well as a pharmaceutical and agricultural intermediate. It is used in the synthesis of metabolites of carcinogenic PAHs. It is also used in the preparation of steroidal antiestrogens through cyclic condensation. Ethyl Bromoacetate is a reactant in the preparation of antim icrobial and antioxidant coumarinyloxymethyl-thiadiazolone.
Preparation
Ethyl bromoacetate is commonly synthesized by sulfuric acid catalyzed esterification of monobromoacetic acid (MBAA). After reaction completion, excess acid is washed out and the product purified via distillation under reduced pressure if necessary (Stenger, 1978; Korhonen, 1984).
Reactions
Ethyl bromoacetate on derivatisation reaction with p-t-butyl calix[4]arene yields 1,3-diester substituted calix[4]arene. It also undergoes Suzuki type cross-coupling reactions with arylboronic acids cocatalyzed by copper(I) oxide.
General Description
Ethyl bromoacetate appears as a clear, colorless liquid. A lachrymator. Toxic by ingestion, inhalation and skin absorption; a strong irritant of the skin. Insoluble in water and soluble in alcohol, benzene, and ether. Specific gravity of 1.5.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Ethyl bromoacetate is a halogenated ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Hazard
Toxic by ingestion, inhalation, and skin absorption; strong irritant.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Bromoacetates and chloroacetates are extremely irritating/lachrymators. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
A poison. An irritant to skin, eyes, and mucous membranes. Questionable carcinogen with experimental neoplastigenic data. Flammable liquid when exposed to heat, flame, and oxidizers. Will react with water or steam to produce toxic and corrosive fumes. To fight fire, use water as a fire blanket. When heated to decomposition or on contact with acid or acid fumes, it emits highly toxic fumes of Br-. See also BROMIDES.
Potential Exposure
Used for making pharmaceuticals; as a warning gas in poisonous, odorless gasses; as a tear gas
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24- 48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, well-ventilated area away from oxidizers, strong acids, strongbases, reducing agents, heat, and sources of ignition. Wherepossible, automatically pump liquid from drums or otherstorage containers to process containers.
Shipping
UN1603 Ethyl bromoacetate, Hazard class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Wash the ester with saturated aqueous Na2CO3 (three times), 50% aqueous CaCl2 (three times) and saturated aqueous NaCl (twice). Dry with MgSO4, CaCl2 or CaCO3, and distil it. [Beilstein 2 IV 527.] LACHRYMATORY.
Incompatibilities
Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, and reducing agents. Esters are generally incompatible with nitrates. Moisture may cause hydrolysis or other forms of decomposition.
Ethyl bromoacetate Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Yancheng Longshen Chemical Co.,Ltd. | +86-0515-88706880; +8618352073383 | sales@longshenchem.com | China | 79 | 55 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12452 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 436 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7038 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9353 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
Related articles
- How to properly handle and store ethyl bromoacetate
- Ethyl bromoacetate, also known as ethyl bromoacetate, is an organic compound with the molecular formula BrCH2COOCH2CH3. It is ....
- Apr 20,2022
View Lastest Price from Ethyl bromoacetate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-23 | Ethyl bromoacetate
105-36-2
|
US $50.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-10-14 | Ethyl bromoacetate
105-36-2
|
US $0.00 / kg | 1kg | 0.99 | 20tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-10-08 | Ethyl bromoacetate
105-36-2
|
US $30.00 / KG | 1KG | 99% | 20T | Firsky International Trade (Wuhan) Co., Ltd |
-

- Ethyl bromoacetate
105-36-2
- US $50.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Ethyl bromoacetate
105-36-2
- US $0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Ethyl bromoacetate
105-36-2
- US $30.00 / KG
- 99%
- Firsky International Trade (Wuhan) Co., Ltd
105-36-2(Ethyl bromoacetate)Related Search:
1of4