1,2-Dibromoethane
- CAS No.
- 106-93-4
- Chemical Name:
- 1,2-Dibromoethane
- Synonyms
- EDB;DIBROMOETHANE;ETHYLENE DIBROMIDE;1,2-dibromethane;1,2-EDB;1,2-Dibromethan;1,2-DIBROMOETANE;ETHYLENE BROMIDE;Soilbrom;CH2BrCH2Br
- CBNumber:
- CB6852689
- Molecular Formula:
- C2H4Br2
Lewis structure
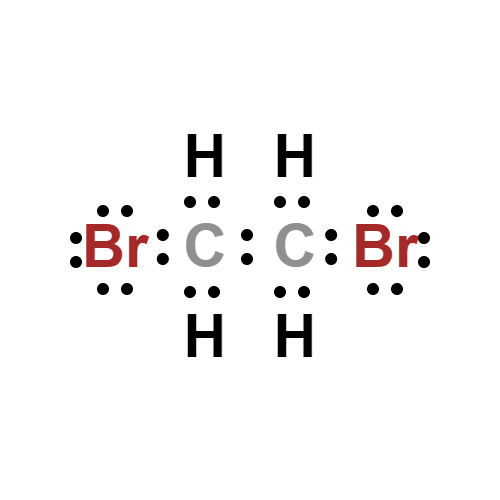
- Molecular Weight:
- 187.86
- MDL Number:
- MFCD00000233
- MOL File:
- 106-93-4.mol
- MSDS File:
- SDS
| Melting point | 9 °C |
|---|---|
| Boiling point | 131-132 °C(lit.) |
| Density | 2.18 g/mL at 25 °C(lit.) |
| vapor density | ~6.5 (vs air) |
| vapor pressure | 11.7 mm Hg ( 25 °C) |
| refractive index |
n |
| Flash point | 132°C |
| storage temp. | 0-6°C |
| solubility | water: soluble250 part |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Odor | Mild, sweet odor detectable at 10 ppm |
| Odor Threshold | 10 ppm |
| Water Solubility | 4 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,3796 |
| BRN | 605266 |
| Henry's Law Constant | 7.58(x 10-4 atm?m3/mol) at 25 °C (static headspace-GC, Welke et al., 1998) |
| Dielectric constant | 4.75 |
| Exposure limits | NIOSH REL: TWA 0.045 ppm, 15-min C 0.13 ppm, IDLH 100 ppm; OSHA PEL: TWA 20 ppm, C 30 ppm, 5-min peak 50 ppm;ACGIH TLV: suspected human carcinogen. |
| Stability | Stable, but may be light sensitive. Incompatible with strong oxidizing agents, magnesium, alkali metals. |
| EPA Primary Drinking Water Standard | MCL:5e-005,MCLG:zero |
| FDA 21 CFR | 165.122 |
| CAS DataBase Reference | 106-93-4(CAS DataBase Reference) |
| EWG's Food Scores | 9 |
| FDA UNII | 1N41638RNO |
| Proposition 65 List | Ethylene dibromide |
| IARC | 2A (Vol. 15, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Ethane, 1,2-dibromo-(106-93-4) |
| EPA Substance Registry System | Ethylene dibromide (106-93-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H315-H319-H335-H350-H411 | |||||||||
| Precautionary statements | P273-P280-P301+P310-P302+P352+P312-P304+P340+P311-P305+P351+P338 | |||||||||
| Hazard Codes | T,N,F | |||||||||
| Risk Statements | 45-23/24/25-36/37/38-51/53-34-39/23/24/25-11 | |||||||||
| Safety Statements | 53-45-61-36/37/39-26-36/37-16-7 | |||||||||
| RIDADR | UN 1605 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KH9275000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29337100 | |||||||||
| Toxicity | LD50 i.p. in mice: 220 mg/kg (Fischer) | |||||||||
| IDLA | 46 ppm (354 mg/m3) | |||||||||
| NFPA 704 |
|
1,2-Dibromoethane price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 48880-U | 1,2-Dibromoethane solution certified reference material, 200?μg/mL in methanol | 106-93-4 | 1mL | $31.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 240656 | 1,2-Dibromoethane ≥99% | 106-93-4 | 5G | $37.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 03505 | 1,2-Dibromoethane purum, ≥98.0% (GC) | 106-93-4 | 100ml | $44.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 03505 | 1,2-Dibromoethane purum, ≥98.0% (GC) | 106-93-4 | 1l | $139 | 2024-03-01 | Buy |
| Alfa Aesar | A12766 | 1,2-Dibromoethane, 99% | 106-93-4 | 250g | $45.1 | 2023-06-20 | Buy |
1,2-Dibromoethane Chemical Properties,Uses,Production
Description
Ethylene dibromide is a colorless nonflammable liquid or solid (below 10℃) with a sweet, chloroformlike odor. The minimum concentration detectable by odor is10 ppm. Molecular weight = 187.88; Specific gravity(H2O:1) = 2.17; Boiling point = 131℃; Freezing/Meltingpoint = 10℃; Relative vapor density (air = 1) = 6.5; Vaporpressure = 12 mmHg. Soluble in water
Chemical Properties
1,2-Dibromoethane is a colorless nonflammable liquid with a mild sweet odor, like chloroform. The minimum concentration detectable by odor is 10 ppm.It is stable at room temperature, but can be slowly decomposed into toxic substances under light. It is miscible with ethanol, ether, carbon tetrachloride, benzene, gasoline and other organic solvents, and forms azeotropes, and dissolves in about 250 times of water. Noncombustible. Very toxic by inhalation, skin absorption or ingestion. used as a solvent, scavenger for lead in gasoline, grain fumigant and in the manufacture of other chemicals.
Physical properties
Colorless liquid with a sweet, chloroform-like odor. Odor threshold concentration is 25 ppb (quoted, Keith and Walters, 1992).
Uses
1,2-Dibromoethane (EDB) is used as a fumigant for grains, in antiknock gasolines, as asolvent, and in organic synthesis. Most of the uses of 1,2-dibromoethane have been stopped in the United States; however, it is still used as a fumigant for treatment of logs for termites and beetles, for the control of moths and beehives, and as a preparation for dyes and waxes.
Uses
Historically, the primary use of 1,2-dibromoethane has been as a lead scavenger in antiknock mixtures added to gasolines (IPCS 1996). Lead scavenging agents transform the combustion products of tetraalkyl lead additives to forms that are more likely to be vaporized from engine surfaces. In 1978, 90% of the 1,2-dibromoethane produced was used for this purpose (ATSDR 1992). Annual consumption of 1,2-dibromoethane in the United States has decreased since the U.S. Environmental Protection Agency banned the use of lead in gasoline.
Preparation
1,2-Dibromoethane is manufactured via uncatalyzed, liquid-phase bromination of ethylene. Gaseous ethylene is brought into contact with bromine by various methods, allowing for dissipation of the heat of the reaction.
Definition
ChEBI: 1,2-dibromoethane is a bromoalkane that is ethane carrying bromo substituents at positions 1 and 2. It is produced by marine algae. It has a role as a fumigant, a carcinogenic agent, a marine metabolite, an algal metabolite, a mouse metabolite and a mutagen. It is a bromohydrocarbon and a bromoalkane.
General Description
1,2-dibromomethane is a heavy, colourless liquid with a mild sweet odour, like chloroform. Ethylene dibromide is incompatible with strong oxidisers, magnesium, alkali metals, and liquid ammonia. Ethylene dibromide is soluble in alcohols, ethers, acetone, benzene, and most organic solvents and slightly soluble in water. It reacts with lead residues to generate volatile lead bromides. Because of limitations in epidemiological study evidences for ethylene dibromide as a human carcinogen is inconclusive. In 1984, the U.S. EPA imposed a ban on its use as a soil and grain fumigant.
Air & Water Reactions
Slightly soluble in water. May react slowly with moisture.
Reactivity Profile
1,2-Dibromoethane slowly decomposes in the presence of light and heat. Turns brown upon exposure to light. Corrosive to iron and other metals. May decompose upon contact with alkalis. Incompatible with oxidizing agents. Reacts with sodium, potassium, calcium, powdered aluminum, zinc, magnesium and liquid ammonia. May attack some plastics, rubber and coatings. May poison platinum catalysts [Hawley]. Reacts as an alkylating agent .
Hazard
Probable carcinogen. Toxic by inhalation, ingestion, and skin absorption; strong irritant to eyes and skin.
Health Hazard
1,2-Dibromoethane is toxic by inhalation,ingestion, or skin contact. The acute toxicsymptoms are depression of the central ner vous system, irritation and congestion oflungs, hepatitis, and renal damage. Chronicexposure can produce conjunctivitis, bron chial irritation, headache, depression, lossof appetite, and loss of weight. Recoveryoccurs after cessation of exposure. Prolongedor repeated exposures to high concentrationscan be fatal to animals and humans. Lethalconcentration for a 2-hour exposure period is400 ppm in rats.
1,2-Dibromoethane is moderate to highlytoxic by ingestion. Its toxicity is far greaterthan that of 1,2-dichloroethane. An oralintake of 5 to 10 mL of the liquid can be fatalto humans. Death occurs from necrosis of theliver and kidney damage. The oral LD50 val ues varied between 50 and 125 mg/kg fordifferent species of laboratory animals.
Vapors are irritant to the eyes. Contactwith the liquid can damage vision. Skincontact may produce severe irritation andblistering.
Mutagenic tests were positive, while thehistidine reversion–Ames test gave incon clusive results (NIOSH 1986). 1,2-Dibromo ethane is carcinogenic to animals and issuspected to cause cancer in humans. Inhala tion of this compound produced tumors inthe lungs and nose in mice and rats. Oraladministration caused cancers in the liver andgastrointestinal tract.
Health Hazard
Local inflammation, blisters and ulcers on skin; irritation in lungs and organic injury to liver and kidneys; may be absorbed through skin.
Flammability and Explosibility
Ethylene dibromide is a noncombustible substance (NFPA rating = 0).
Agricultural Uses
Fumigant, Nematicide: Not approved for use in EU countries. Not registered for use in the U.S. Persons whose clothing or skin is contaminated with liquid ethylene dibromide (above 10°C) can secondarily contaminate others by direct contact or through off-gassing vapor. Ethylene dibromide was used extensively as a pesticide and an ingredient of soil, vegetable, fruit, and grain fumigant formulations. Still used in India, South Africa and other countries. There are 15 global suppliers.
Trade name
AADIBROOM®; EDB-85; FUMO-GAS®; ISCOBROME D®; KOPFUME®; NEFIS®; NEPHIS®; SOILFUME®; UNIFUME®
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and teratogenic data. Human poison by ingestion. Experimental poison by ingestion, sktn contact, intraperitoneal, and possibly other routes. Moderately toxic by inhalation and rectal routes. Human systemic effects by ingestion: hypermothty, barrhea, nausea or vomiting, decreased urine volume or anuria. Experimental reproductive effects. Human mutation data reported. A severe skin and eye irritant. Implicated in worker sterdity. When heated to decomposition it emits toxic fumes of Br-. See also ETHYLENE DICHLORIDE and BROMIDES.
Potential Exposure
Ethylene dibromide is used as a chemical intermediate; as a fumigant for ground pest control; as a constituent of ethyl gasoline (anti-knock agent). It is also used in fire extinguishers, gauge fluids, and waterproofing preparations; and it is used as a solvent for celluloid, fats, oils, and waxes. Pesticide not in use; TRI and/or IUR indicates importers or manufacturers are unlikely
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24- 48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray
Carcinogenicity
1,2-Dibromoethane is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Cancer Studies in Experimental Animals
Metabolic pathway
The bacterial strain GP1 can utilize 1,2-dibromoethane as a sole carbon and energy source. The first step in 1,2-dibromoethane is catalyzed by a hydrolytic haloalkane dehalogenase and the resulting 2- bromoethanol is rapidly converted to ethylene oxide, preventing the accumulation of 2-bromoethanol and 2- bromoacetaldehyde. However, the further metabolic pathway(s) is unclear.
storage
work with EDB should be conducted in a fume hood to prevent exposure by inhalation, and appropriate impermeable gloves and safety goggles should be worn to prevent skin contact. Gloves and protective clothing should be changed immediately if EDB contamination occurs. Since EDB can penetrate neoprene and other plastics, protective apparel made of these materials does not provide adequate protection from contact with EDB.
Shipping
UN1605/154 Ethylene dibromide, Hazard Class: 6.1; Labels: 6.1-Poison Inhalation Hazard, Inhalation Hazard Zone B
Purification Methods
Wash the dibromide with conc HCl or H2SO4, then water, aqueous NaHCO3 or Na2CO3, more water, and dry it with CaCl2. Fractionally distil it. Alternatively, keep in daylight with excess bromine for 2hours, then extract with aqueous Na2SO3, wash with water, dry with CaCl2, filter and distil. It can also be purified by fractional crystallisation by partial freezing. Store it in the dark. [Beilstein 1 H 90, 1 I 28, 1 II 61, 1 III 182, 1 IV 158.]
Incompatibilities
Reacts vigorously with chemically active metals; liquid ammonia, strong bases; strong oxidizers; causing fire and explosion hazard. Light, heat, and moisture can cause slow decomposition, forming hydrogen bromide. Attacks fats, rubber, some plastics and coatings.
Waste Disposal
Controlled incineration with adequate scrubbing and ash disposal facilities
1,2-Dibromoethane Preparation Products And Raw materials
Raw materials
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 436 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Yancheng Longshen Chemical Co.,Ltd. | +86-0515-88706880; +8618352073383 | sales@longshenchem.com | China | 79 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10248 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
Related articles
- Related uses of 1,2-Dibromoethane
- 1,2-Dibromoethane [106-93- 4], BrCH2CH2Br (ethylene dibromide), is manufactured by the uncatalyzed addition of bromine to ethy....
- Oct 21,2023
- Applications of 1,2-Dibromoethane
- 1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula (CH2Br2). It....
- Nov 11,2019
View Lastest Price from 1,2-Dibromoethane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-26 | 1,2-Dibromoethane
106-93-4
|
US $100.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-10-08 | 1,2-Dibromoethane
106-93-4
|
US $30.00 / KG | 1KG | 99% | 20T | Firsky International Trade (Wuhan) Co., Ltd | |
 |
2023-09-06 | 1,2-Dibromoethane
106-93-4
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- 1,2-Dibromoethane
106-93-4
- US $100.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- 1,2-Dibromoethane
106-93-4
- US $30.00 / KG
- 99%
- Firsky International Trade (Wuhan) Co., Ltd
-

- 1,2-Dibromoethane
106-93-4
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.





