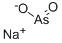Sodium m-arsenite
- CAS No.
- 7784-46-5
- Chemical Name:
- Sodium m-arsenite
- Synonyms
- atlas;penite;sodanit;atlas"a";atlas’a’;kill-all;chempelsc;chem-sen56;prodalumnol;ARSENIC METAL
- CBNumber:
- CB6854335
- Molecular Formula:
- AsO2.Na
- Molecular Weight:
- 129.91
- MDL Number:
- MFCD00085309
- MOL File:
- 7784-46-5.mol
| Melting point | 817 °C(lit.) |
|---|---|
| Boiling point | 613 °C(lit.) |
| Density | 5.727 g/mL at 25 °C(lit.) |
| storage temp. | Store at +15°C to +25°C. |
| solubility | H2O: soluble |
| form | powder |
| color | off-white to gray |
| PH | 9.3 (H2O, 20°C) |
| Water Solubility | Miscible with water. Slightly miscible with alcohol. |
| Merck | 13,8651 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. Absorbs carbon dioxide from air. |
| FDA UNII | 48OVY2OC72 |
| EPA Substance Registry System | Sodium arsenite (7784-46-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H350-H410 | |||||||||
| Precautionary statements | P201-P202-P261-P273-P301+P310-P304+P340+P311 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 23/25-50/53-51/53-45-52/53-22-23/24/25-21-34 | |||||||||
| Safety Statements | 20/21-28-45-60-61-53-36/37-36/37/39-26 | |||||||||
| RIDADR | UN 1558 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CG0525000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2842 90 80 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in rats: 0.041 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Sodium m-arsenite Chemical Properties,Uses,Production
Chemical Properties
Sodium arsenite is a white or grayish-white powder or flakes.
Chemical Properties
white granular crystals
Uses
Sodium arsenite is used as hide preservative, antiseptic, dyeing and soaps. It is used as a stressor to induce production of heat shock proteins.
Uses
Technical grade in manufacture of arsenical soap for use on skins, for treating vines against certain scale diseases; as insecticide especially for termites.
Definition
ChEBI: An inoganic sodium salt with formula with formula NaAsO2.
General Description
A white or grayish-white powder. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption.
Air & Water Reactions
Soluble in water. Slowly converted in solution to arsenates by atmospheric oxygen; in dry state Sodium m-arsenite is decomposed by carbon dioxide. [EPA, 1998].
Reactivity Profile
Salts, basic, such as SODIUM ARSENITE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Toxic by ingestion and inhalation.
Health Hazard
Extremely toxic: probable oral lethal dose (human) 5-50 mg/kg, between 7 drops and one teaspoon for 70 kg person (150 lb.). Poisonous if swallowed or inhaled. Human suspected carcinogen.
Fire Hazard
Sodium m-arsenite may burn but does not ignite readily. When heated Sodium m-arsenite emits toxic fumes of arsenic and sodium oxide. Slowly converted in solution to arsenates by atmospheric oxygen; in dry state Sodium m-arsenite is decomposed by carbon dioxide.
Safety Profile
Confirmed human carcinogen. Human poison by ingestion. Experimental poison by ingestion, skin contact, intravenous, intramuscular, and intraperitoneal routes. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. Used as an herbicide and pesticide. When heated to decomposition it emits toxic fumes of arsenic and NazO. See also ARSENIC COMPOUNDS.
Potential Exposure
This material is used in manufacturing of arsenical soap for use on skin; treating vines against certain scale diseases; wood preservation; as a reagent in preparation of Methylene iodide; corrosion inhibitor; and for herbicidal and pesticidal purposes
Shipping
UN2027 Sodium arsenite, solid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN1686 Sodium arsenite, aqueous solutions, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Chemically active metals. Arsine, a very deadly gas, can be released in the presence of acid, acid mists, or hydrogen gas.
Waste Disposal
The arsenic may be precipitated as calcium arsenite. It should be stored until recycled. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.