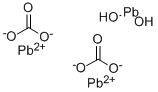
Lead(II) carbonate basic
- CAS No.
- 1319-46-6
- Chemical Name:
- Lead(II) carbonate basic
- Synonyms
- LEAD CARBONATE;basicleadcarbonate;LEAD(II) CARBONATE;LEAD(II) CARBONATE, BASIC;carbonicacid,leadsalt,basic;ceruse;cerussa;White 1;flakelead;C.I.77597
- CBNumber:
- CB7106691
- Molecular Formula:
- 2CO3.2Pb.H2O2Pb
- Molecular Weight:
- 775.63
- MDL Number:
- MFCD00078155
- MOL File:
- 1319-46-6.mol
- MSDS File:
- SDS
| Melting point | 400 °C (dec.)(lit.) |
|---|---|
| Density | 6,14 g/cm3 |
| solubility | insoluble in H2O, ethanol; soluble in acid solutions |
| form | Powder |
| Specific Gravity | 6.14 |
| color | White to off-white |
| Water Solubility | Insoluble |
| Solubility Product Constant (Ksp) | pKsp: 13.13 |
| Exposure limits |
ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 1319-46-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | WDF96425HV |
| EPA Substance Registry System | Basic lead carbonate (1319-46-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H332-H360FD-H373-H410 | |||||||||
| Precautionary statements | P202-P260-P273-P301+P312-P304+P340+P312-P308+P313 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 61-20/22-33-50/53-62 | |||||||||
| Safety Statements | 53-45-60-61 | |||||||||
| RIDADR | UN 2291 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OF9275000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28369917 | |||||||||
| NFPA 704 |
|
Lead(II) carbonate basic price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1.07381 | Lead(II) carbonate for analysis EMSURE? ACS | 1319-46-6 | 250g | $176.67 | 2021-12-16 | Buy |
| Alfa Aesar | 042089 | Lead(II) carbonate, basic, 99% (metals basis) | 1319-46-6 | 2kg | $464 | 2023-06-20 | Buy |
| Alfa Aesar | 033328 | Lead(II) carbonate, basic, ACS | 1319-46-6 | 250g | $112 | 2023-06-20 | Buy |
| Sigma-Aldrich | 243582 | Lead(II) carbonate basic 325 mesh | 1319-46-6 | 100g | $19.68 | 2024-03-01 | Buy |
| Sigma-Aldrich | 243582 | Lead(II) carbonate basic 325 mesh | 1319-46-6 | 2.5kg | $318 | 2024-03-01 | Buy |
Lead(II) carbonate basic Chemical Properties,Uses,Production
Solubility in water(g/100ml)
Dissolved grams per 100 milliliters of water:
7.269 × 10-5/20 °C Toxicity
Chemical properties
White powder, hexagonal crystal system. It is insoluble in water and ethanol, and soluble in acetic acid and nitric acid. Gamma-Butyrolactone
Uses
Lead carbonate basic has a high refractive index and weather fastness, widely used in pigments, coatings, plastics, printing and dyeing, analysis reagents, etc .; for pigments, it is used for excellent pearlescent pigments; and it is also used to produce inorganic pigments with paint and pigments. In the printing and ink, it is used for packaging paper, business cards, plastic cloth, textiles and so on. As the possibility of leading to lead poisoning in the production and application, and the vulnerability to thicken when the paint is made by white lead, and the whiteness easily declining when contact with and hydrogen sulfide and other shortcomings, its application is limited. While the coating film produced by the lead white is more stable, with excellent weather resistance and rust resistance. In the paint industry, it is still used as the white pigments for the production of the original paint, anti-rust paint and outdoor paint; raw materials for ceramic glaze, painting and cosmetic; amino or acrylic baking finish of coating. Add 2% to 8%. It is used in painting with cars, motorcycles, bicycles, furniture and so on. In plastic, basic lead carbonate can be used as vinyl chloride plastic stabilizer for the production of pearlescent plastic, pearlescent paint and so on.
Preparation
1. Chemical method: adding carbon dioxide, deionized water into reaction solution prepared by lead acetate, lead oxide and deionized water. After reaction, then by precipitation, added with nitrocellulose pulp slurry, precipitation, crystallization, centrifugal dehydration, washed with alcohol, drying, the basic lead carbonate is obtained. Alcohol waste liquid is treated and recovered. The reaction equation is: Pb(Ac)2+PbO+H2O→Pb(Ac)2•Pb(OH)2;
3[Pb(Ac)2•Pb(OH)2]+2CO2→3Pb(Ac)2+2PbCO3•Pb(OH)2+2H2O
2. Acetic acid method: mix yellow lead, acetic acid and water together in the mother liquor tank, and the concentration of the mixture of lead oxide is 230~250 g/L. Suspend the mixture with stirring; keep warm for 3h in the 90 ℃ leading to the formation of basic lead acetate; clarify and carbonize with purified carbon dioxide; when 85% lead hydroxide in the basic lead acetate is carbonized, that is the end of the reaction; and then by precipitation, centrifugal separation, with oil and alcohol washing and drying, basic lead carbonate products is made. In addition, the separated mother liquor can be recycled. The reaction equation is: 2PbO + 2HAc → Pb (Ac) 2 • Pb (OH) 2
3[Pb(Ac)2•Pb(OH)2]+2CO2→3Pb(Ac)2+2PbCO3•Pb(OH)2+2H2O.
Toxicity
Early symptoms of poisoning is that lead linear appearing in the edge of the gums and urinary poisoning. For chronic poisoning, it has changes in the nervous system, the emergence of debilitating syndrome, encephalopathy, dysmotility, changes in the blood system, metabolic and endocrine disorders, gastrointestinal changes and changes in the cardiovascular system.
A maximum allowable concentration of lead and lead inorganic compounds is 0.01 mg/m3 and 0.0007 mg/m3 in an average working day.
When the poisoned suffered colic, he should receive subcutaneous injection of atropine, morphine, and intravenous injection of magnesium sulfate, sodium thiosulfate, calcium chloride. Masks blocking 95% to 97% of lead dust is allowable when working; when in the environment of high vapor concentration, you can use the filter gas mask for a forced supply of fresh air. When the poisoned ones suffered colic, he should receive subcutaneous injection of atropine, morphine, and intravenous injection of magnesium sulfate, sodium thiosulfate, calcium chloride.
Chemical Properties
Basic lead carbonate forms white hexagonal crystals; it decomposes when heated to 400 °C. It is insoluble in water and alcohol, slightly soluble in carbonated water, and soluble in nitric acid.
Chemical Properties
dense white powder
Uses
Lead(II) carbonate is used in ceramic glaze and exterior paint.
Uses
Lead(II) carbonate basic can be used as pigment in oil paints and water colors; in cements; for making putty and lead carbonate paper; in the processing of parchment.
Definition
Acid-soluble, heavy, white powder or crystalline substance, insoluble in water and alco- hol.
Production Methods
Basic Lead Carbonate is produced by several methods, in which soluble lead acetate is treated with carbon dioxide. For example, in the Thompson-Stewart process, an aqueous slurry of finely divided lead metal or monoxide, or a mixture of both, is treated with acetic acid in the presence of air and carbon dioxide. High quality, very fine particle-size basic lead carbonate is produced, ranging in carbonate content from 62 to 65% (vs 68.9% PbCO3, theoretical).
Preparation
Many commercial processes have been developed for manufacturing basic lead carbonate. These include: Thomson-Stewart process, Carter process, and Dutch process. The method of preparation involves treating lead with acetic acid vapors in the presence of carbon dioxide at 60°C. In the Thomson-Stewart process, finely divided lead monoxide or lead metal is mixed with water to give aqueous slurry, which is then mixed with acetic acid in the presence of air and carbon dioxide. All these processes are slow, taking weeks to obtain products of desired composition.
Basic lead carbonate also is precipitated by dissolving lead monoxide in lead(II) acetate solution, and treating the solution with carbon dioxide. It also is produced by electrolysis of sodium nitrate or sodium acetate using lead anode and then precipitating out the product by adding sodium carbonate.
.
Flammability and Explosibility
Non flammable
Lead(II) carbonate basic Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 | sales@simchoicechem.com | China | 255 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
View Lastest Price from Lead(II) carbonate basic manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-12 | Lead(II) carbonate basic
1319-46-6
|
US $0.00 / kg | 1kg | 99% | 2000ton | Shaanxi Haibo Biotechnology Co., Ltd | |
 |
2023-09-21 | Lead(II) carbonate basic
1319-46-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-07-28 | Lead(II) carbonate basic
1319-46-6
|
US $100.00-50.00 / KG | 1KG | 99% | 10000kg | Hebei Guanlang Biotechnology Co,.LTD |
-

- Lead(II) carbonate basic
1319-46-6
- US $0.00 / kg
- 99%
- Shaanxi Haibo Biotechnology Co., Ltd
-

- Lead(II) carbonate basic
1319-46-6
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Lead(II) carbonate basic
1319-46-6
- US $100.00-50.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co,.LTD