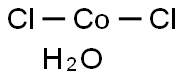Cobalt chloride hexahydrate
- CAS No.
- 7791-13-1
- Chemical Name:
- Cobalt chloride hexahydrate
- Synonyms
- COCl;COBALT(II) CHLORIDE HEXAHYDRATE;cobalt chloride hydrate;COBALTOUS CHLORIDE HEXAHYDRATE;COBALTOUS CHLORIDE;chlorekcobaltawy;Cobalt chlordie;CobaltousChlorideAr;COBALT CHLORIDE XTL;COBALT CHLORIDE, ACS
- CBNumber:
- CB7226636
- Molecular Formula:
- Cl2CoH2O
- Molecular Weight:
- 147.85
- MDL Number:
- MFCD00149652
- MOL File:
- 7791-13-1.mol
- MSDS File:
- SDS
| Melting point | 86 °C |
|---|---|
| Boiling point | 1049 °C |
| Density | 3.35 |
| vapor pressure | 40 mm Hg ( 0 °C) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 191g/l |
| form | Solid |
| Specific Gravity | 1.924 |
| color | Red-purple |
| PH | 4.9 (50g/l, H2O, 25℃) |
| Odor | at 100.00?%. odorless |
| Water Solubility | Soluble in water, ether, acetone and alcohol. |
| Sensitive | Hygroscopic |
| Merck | 14,2437 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability | Hygroscopic |
| LogP | -1.380 (est) |
| CAS DataBase Reference | 7791-13-1(CAS DataBase Reference) |
| FDA UNII | 17AVG63ZBC |
| EPA Substance Registry System | Cobalt chloride (CoCl2), hexahydrate (7791-13-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H317-H334-H341-H350i-H360FD-H410 | |||||||||
| Precautionary statements | P202-P273-P280-P301+P312-P302+P352-P308+P313 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 49-22-42/43-50/53-68-60 | |||||||||
| Safety Statements | 53-22-45-60-61 | |||||||||
| RIDADR | UN 3260 8/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | GG0200000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28273400 | |||||||||
| Toxicity | LD50 orally in rats: 766 mg/kg (Speijers) | |||||||||
| NFPA 704 |
|
Cobalt chloride hexahydrate price More Price(71)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 202185 | Cobalt(II) chloride hexahydrate reagent grade | 7791-13-1 | 25g | $33.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 202185 | Cobalt(II) chloride hexahydrate reagent grade | 7791-13-1 | 1kg | $288 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02539 | Cobalt(II) chloride hexahydrate for analysis EMSURE? ACS,Reag. Ph Eur | 7791-13-1 | 100g | $394 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02539 | Cobalt(II) chloride hexahydrate for analysis EMSURE? ACS,Reag. Ph Eur | 7791-13-1 | 250g | $656 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02533 | Cobalt(II) chloride hexahydrate extra pure | 7791-13-1 | 1KG | $580 | 2022-05-15 | Buy |
Cobalt chloride hexahydrate Chemical Properties,Uses,Production
Physical Properties
Blue leaflets; turns pink in moist air; hygroscopic; the dihydrate is violet blue crystal; the hexahydrate is pink monoclinic crystal; density 3.36, 2.48 and 1.92 g/cm3 for anhydrous salt, dihydrate and hexahydrate, respectively; anhydrous salt melts at 740°C and vaporizes at 1,049°C; vapor pressure 60 torr at 801°C; the hexahydrate decomposes at 87°C; the anhydrous salt and the hydrates are all soluble in water, ethanol, acetone, and ether; the solubility of hydrates in water is greater than the anhydrous salt.
Uses
Cobalt(II) chloride has several applications. It is used in hygrometers; as a humidity indicator; as a temperature indicator in grinding; as a foam stabilizer in beer; in invisible ink; for painting on glass; in electroplating; and a catalyst in Grignard reactions, promoting coupling with an organic halide. It also is used to prepare several other cobalt salts; and in the manufacture of synthetic vitamin B12.
Preparation
Cobalt(II) chloride is prepared by the action of cobalt metal or its oxide, hydroxide, or carbonate with hydrochloric acid:
Co(OH)2 + 2HCl → CoCl2 + 2H2O
The solution on concentration and cooling forms crystals of hexahydrate which on heating with SOCl2 dehydrates to anhydrous cobalt(II) chloride. Alternatively, the hexahydrate may be converted to anhydrous CoCl2 by dehydration in a stream of hydrogen chloride and dried in vacuum at 100–150°C.
The anhydrous compound also may be obtained by passing chlorine over cobalt powder.
Reactions
Cobalt(II) chloride undergoes many double decomposition reactions in aqueous solution to produce precipitates of insoluble cobalt salts. For example, heating its solution with sodium carbonate yields cobalt(II) carbonate:
CoCl2 + Na2CO3 CoCO3 + 2NaCl
Reaction with alkali hydroxide produces cobalt(II) hydroxide:
CoCl2 + 2NaOH → Co(OH)2 + 2NaCl
Reaction with ammonium hydrogen phosphate yields cobalt(II) phosphate:
3CoCl2 + 2(NH4)2HPO4 → Co3(PO4)2 +4NH4Cl + 2HCl
While cobalt(II) fluoride is the product of the reaction of anhydrous cobalt(II) chloride with hydrofluoric acid, cobalt(III) fluoride is obtained from fluorination of an aqueous solution of cobalt(II) chloride.
Addition of potassium nitrite, KNO2 to a solution of cobalt(II) chloride yields yellow crystalline potassium hexanitrocobaltate(III), K3Co(NO2)6.
Toxicity
The compound is toxic at high doses. Symptoms include chest pain, cutaneous flushing, nausea, vomiting, nerve deafness, and congestive heart failure. The systemic effects in humans from ingestion include anorexia, increased thyroid size, and weight loss (Lewis (Sr.), R. J. 1996. Sax’s Dangerous Properties of Industrial Materials, 9th ed. New York: Van Nostrand Reinhold). Ingestion of a large amount (30–50 g) could be fatal to children.
Chemical Properties
Cobalt(II) chloride hexahydrate takes the form of purple crystals. Upon heating, it loses its water of crystallisation and decomposes into blue crystals of anhydrous cobalt(II) chloride. Soluble in water, soluble in ethanol, acetone and ether.
Cobalt chloride hexahydrate is utilized in commercial applications such as electroplating, catalyst preparation, painting on glass and porcelain, and in the manufacture of vitamin B12.
Uses
Cobalt(II) chloride hexahydrate (CoCl2·6H2O) has been found to be an efficient catalyst for the one-pot synthesis of biscoumarin derivatives through a combination of aromatic aldehydes and 4-hydroxycoumarin in aqueous media at 70°C. Cobalt chloride hexahydrate can be used as an inducer of HIF-1 production used to study apoptotic effects in HepG2 cells. It is also used as APHA color standard.
Application
Cobalt chloride hexahydrate can be used as Invisible ink; humidity and water indicator; in hygrometers; tempereture indicator in grinding; in electroplating; for painting on glass and porcelain; preparation of catalysts; fertilizer and feed additive; foam stabilizer in beer; as absorbent for military poison gas and ammonia; in manufacture of vitamin B12.
Definition
ChEBI: Cobalt chloride hexahydrate is a hydrate of cobalt chloride containing cobalt (in +2 oxidation state), chloride and water moieties in the ratio 1:2:6. It has a role as an allergen. It contains a cobalt dichloride.
General Description
Cobalt(II) chloride hexahydrate has a cobalt ion surrounded by a tetragonal arrangement of chloride ligands. Four water molecules occupy a square plane about cobalt while chloride ions occupy axial positions. Its crystals belong to the monoclinic lattice system.
Purification Methods
A saturated aqueous solution at room temperature is fractionally crystallised by standing overnight. The first half of the material that crystallises in this way is used in the next crystallisation. The process is repeated several times, water being removed in a dry-box using air filtered through glass wool and dried over CaCl2 [Hutchinson J Am Chem Soc 76 1022 1954]. It has also been crystallised from dilute aqueous HCl. The hexahydrate m 86o forms pink to red deliquescent crystals. It loses 4H2O on heating at 52-56o and forms the violet dihydrate which loses a further H2O at 100o to form the violet monohydrate which loses the last H2O at 120-140o to give the pale blue anhydrous deliquescent salt m 735o and b 1049o. A pink solution of CoCl2 in H2O becomes blue on heating to 50o or adding conc HCl which may precipitate the mono or dihydrate. The solid dihydrate gives a blue-purple solution with EtOH. Note: CoCl2 in H2O is a “sympathetic ink”, i.e. writing using an aqueous solution is almost invisible on paper, but becomes blue on warming the paper. On cooling or standing, the writing becomes invisible again. The anhydrous salt is soluble in H2O, EtOH, Et2O, Me2CO and pyridine. [Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1515 1965.]
Structure and conformation
The hexahydrate COCl2.6H2O forms monoclinic crystals in which each cobalt atom is surrounded by four water molecules at the corners of a distorted square (Co-H20 = .12) and by two chlorine atoms (Co-Cl = 2.43) to form a distorted octahedron.
The other two water molecules are not bonded directly to cobalt. In the monoclinic dihydrate CoCl2.2H2O, the octahedral symmetry around the cobalt atom is maintained by the sharing of chlorine atoms between two cobalt atoms. In the flattened octahedral structuress there are two Co-Cl distances of 2-450, two of 2.478 and two Co-H2O distances of 2.040.
Cobalt chloride hexahydrate Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7377 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 89 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
Related Qustion
- Q:What is Cobalt chloride hexahydrate?
- A:CoCl2-6H2O can be used as reaction catalyst, paint drying agent, ammonia absorber, neutral dyes, desiccant, ceramic colouring ....
- Apr 19,2024
View Lastest Price from Cobalt chloride hexahydrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-16 | Cobalt Chloride Hexahydrate | US $0.00 / kg | 1kg | 98% | 10T | Yujiang Chemical (Shandong) Co.,Ltd. | |
 |
2024-04-07 | Cobalt chloride hexahydrate
7791-13-1
|
US $0.00 / KG | 25KG | 98% | 50MT/MONTH | Hebei Yanxi Chemical Co., Ltd. | |
 |
2024-03-29 | Cobalt chloride hexahydrate
7791-13-1
|
US $6.00-0.80 / kg | 1kg | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd |
-

- Cobalt Chloride Hexahydrate
- US $0.00 / kg
- 98%
- Yujiang Chemical (Shandong) Co.,Ltd.
-

- Cobalt chloride hexahydrate
7791-13-1
- US $0.00 / KG
- 98%
- Hebei Yanxi Chemical Co., Ltd.
-

- Cobalt chloride hexahydrate
7791-13-1
- US $6.00-0.80 / kg
- 99%
- Henan Fengda Chemical Co., Ltd