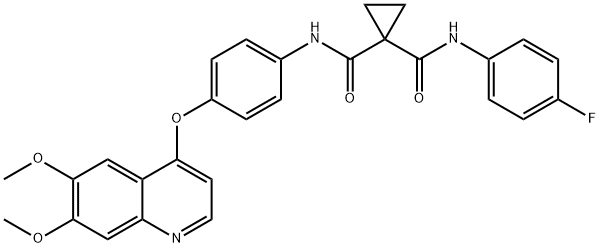
Cabozantinib
- CAS No.
- 849217-68-1
- Chemical Name:
- Cabozantinib
- Synonyms
- XL184;Carbozantinib;N-(4-(6,7-diMethoxyquinolin-4-yloxy)phenyl)-N-(4-fluorophenyl)cyclopropane-1,1-dicarboxaMide(CAS:849217-68-1);CS-6;XL-186;BMS907351;Cabotinib;Cabozantinib;Carbozanitinb;Phloroglucino
- CBNumber:
- CB72525898
- Molecular Formula:
- C28H24FN3O5
- Molecular Weight:
- 501.51
- MDL Number:
- MFCD20926324
- MOL File:
- 849217-68-1.mol
| Melting point | 212-215°C |
|---|---|
| Boiling point | 758.1±60.0 °C(Predicted) |
| Density | 1.396 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO |
| form | White powder. |
| pka | 13.86±0.70(Predicted) |
| color | White |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| FDA UNII | 1C39JW444G |
| ATC code | L01EX07 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H318 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P280-P305+P351+P338-P310 | |||||||||
| NFPA 704 |
|
Cabozantinib price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 18464 | XL184 ≥98% | 849217-68-1 | 1mg | $44 | 2024-03-01 | Buy |
| Cayman Chemical | 18464 | XL184 ≥98% | 849217-68-1 | 5mg | $97 | 2024-03-01 | Buy |
| Cayman Chemical | 18464 | XL184 ≥98% | 849217-68-1 | 10mg | $151 | 2024-03-01 | Buy |
| Cayman Chemical | 18464 | XL184 ≥98% | 849217-68-1 | 50mg | $533 | 2024-03-01 | Buy |
| Tocris | 5422 | XL184 ≥98%(HPLC) | 849217-68-1 | 10 | $221 | 2021-12-16 | Buy |
Cabozantinib Chemical Properties,Uses,Production
Anti-cancer drugs
Cabozantinib is a kind of a novel type of molecular targeted drugs developed by the United States Exelixis biopharmaceutical company. On November 29, 2012, the US Food and Drug Administration (FDA) approved the use of cabozantinib for the treatment of unresectable malignant local advanced or metastatic medullary thyroid carcinoma.
In addition, Sorafenib, Vandernib and Levotinib have been approved by the Food and Drug Administration (FDA) for the treatment of advanced thyroid cancer.
Mechanism of action
In vitro biochemical and/or cytological analysis have shown that cabozantinib can inhibit the tyrosine kinase activity of RET, human hepatocyte growth factor receptor (MET), vascular endothelial growth factor receptor 1 (VEGFR-1, VEGFR-2, VEGFR-3, stem cell factor receptor (KIT), receptor kinase receptor (TRKB), FMS-like tyrosine kinase-3, FLT-3), AXL and human tyrosine kinase with immunoglobulinlike and EGF-like domains 2, TIE-2. All the above kinase receptors play an important role in the growth of normal cells and tumor cells. The abnormal expression of these receptors is critical in the development and progression of many kinds of tumors, including the inhibition of tumor cell apoptosis, participating into the tumor angiogenesis and invasion and other pathological processes. Cabozantinib exerts its antitumor effect by inhibiting the above kinase activity, killing tumor cells, reducing metastasis and inhibiting tumor angiogenesis.
Pharmacokinetics
The pharmacokinetic analysis showed that the half-life of this drug was about 55 h with the volume of distribution (V/F) being about 349L, and the clearance rate of CLL being about 4.4 L • h-1. Upon oral administration of this drug, the time for reaching plasma concentration peak time (tmax) is 2~5h. Compared with a single dose of oral administration of 140 mg • d ^ (-1) for 19 days, the exposure amount in vivo was increased to 4-5 times (based on the area under the drug-time curve) and can reach steady-state in 15th day. The drug has a high binding rate with plasma protein (≥ 99.7%).
For healthy people subjecting to a single oral dose of 140 mg of this drug, a high-fat diet compared with the administration upon empty stomach, the maximal concentration (Cmax) and AUC are increased by 41% and 57%, respectively.
In vitro studies have shown that cabozantinib is a CYP3A4 substrate. The CYP3A4 inhibitor will reduce the formation of its metabolite N-oxide (> 80%) while CYP2A9 inhibitor has a relatively small effect on the drug metabolism (<20%). CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C19, CYP2D6 and CYP2E1 have no effect on the drug metabolism.
After a single dose of radiolabeled cabozantinib was administered to healthy subjects, 27% of the radioactivity appeared in the urine and 54% in the stool.
Side effects
In clinical trials, common adverse effects caused by cabozantinib include diarrhea, stomatitis, palmar-plantarrythrodysesthesia syndrome (PPES), and loss of weight, loss of appetite, nausea, fatigue, oral pain, taste disorders, high blood pressure, abdominal pain and constipation.
The most common laboratory abnormalities (> 25%) were increased aspartate aminotransferase, alanine aminotransferase, lymphopenia, increased alkaline phosphatase level, hypocalcemia, neutropenia, thrombocytopenia, hypophosphatemia and hyperbilirubinemia.
For patients receiving cabozantinib, 57% of patients have gotten increased level of thyroid stimulating hormone after initial dose, compared with patients receiving placebo, increased by 19%.
Information regarding to the anticancer drug, mechanism of action, pharmacokinetics, side effects, and drug interactions of cabozantinib were compiled and edited by Yan Shi from Chemicalbook. (2015-10-23)
Medicine interactions
To healthy subjects, administration of strong CYP3A4 inhibitor, ketoconazole (400 mg • d-1, 27 days) can increase the single dose exposure amount (AUC0-inf) of the drug by 38%. Upon administration of this drug, it should be avoided of taking potent CYP3A4 drugs inhibitors such as ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin and voriconazole).
To healthy subjects, administration of strong CYP3A4 inducing drugs, rifampicin (600mg • d-1, a total of 31 d) can reduce the single dose exposure amount (AUC0-inf) of the drug by 77%. It should be avoided of simultaneously administration CYP3A4 induced drugs (such as dexamethasone, Phenytoin, carbamazepine, rifampicin, rifabutin, rifapentine, phenobarbital and hypericum perforatum preparation).
In the human liver microsomal (HLM) enzyme system, cabozantinib is the noncompetitive inhibitor of CYP2C8, CYP2C9 mixed inhibitor and the weak competitive inhibitor of CYP2C19 and CYP3A4.
In the cultured human hepatocytes, carbotinib is a kind of CYP1A1 mRNA induction drug, but has no effect on CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19 or CYP3A4 mRNA or its isozyme system.
In patients with solid tumors, the steady-state plasma concentration of carbotinib (≥100 mg • d-1 for a total of at least 21 days) showed no significant influence on plasma exposure amount (Cmax and AUC) of single doses of rosiglitazone (CYP2C8 substrate).
Carbotinib is a P-glycoprotein transport inhibitory drug, but is not its substrate. Therefore, carbotinib may increase the plasma concentration of P-glycoprotein substrate.
Description
Cabozantinib was approved inNovember 2012 for the treatment of patients with progressive, unresectable, locally advanced, or metastatic medullary thyroid cancer (MTC). Cabozantinib was granted orphan drug status by the FDA to facilitate development of newtreatment options for patients with MTC. It is a member of a class of tyrosine kinase inhibitors (TKIs) with nanomolar pan-inhibitory activity against VEGFR2, MET, and RET among others. Inhibition of the VEGF pathway has been shown preclinically to initially slow tumor growth, but rapid revascularization is followed by aggressive tumor growth. The MET pathway has been implicated in the development of VEGF resistance, so dual VEGF/MET activity is viewed as desirable. In addition, mutations in RET play a particular role in MTC, with 25% of the tumors inheriting a germlinemutation in the proto-oncogene, so multiple tyrosine kinase inhibition may be viewed as particularly beneficial for the treatment of MTC.
Originator
Exelixis (United States)
Uses
XL184 can be used in biological study. Computational network biological approach based on pathway cross-talk inhibition identified new synergistic drug combinations using raloxifene and cabozantinib for treatment of human breast cancer in xenograft mouse model. Potent c-MET inhibitor.
Uses
Cabozantinib (XL184, BMS-907351) is a potent VEGFR2 inhibitor with IC50 of 0.035 nM and also inhibits c-Met, Ret, Kit, Flt-1/3/4, Tie2, and AXL with IC50 of 1.3 nM, 4 nM, 4.6 nM, 12 nM/11.3 nM/6 nM, 14.3 nM and 7 nM, respectively
Definition
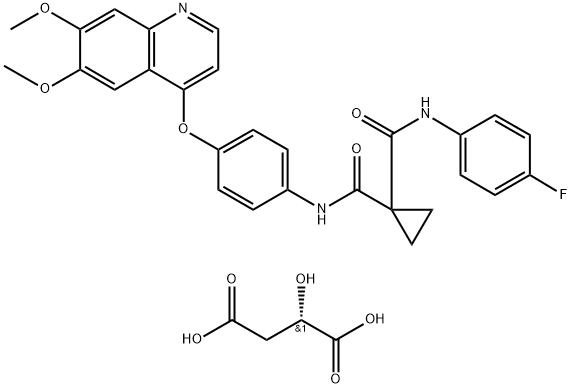
ChEBI: A dicarboxylic acid diamide that is N-phenyl-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide in which the hydrogen at position 4 on the phenyl ring is substituted by a (6,7-dimethoxyquinolin-4-yl)oxy group. A multi-t rosine kinase inhibitor, used (as its malate salt) for the treatment of progressive, metastatic, medullary thyroid cancer.
brand name
Cometriq
Clinical Use
Cabozantinib (PF-06463922; brand name Cabometyx; Exelixis, Alameda, CA) is an oral multikinase inhibitor with CNS penetration. It is FDA approved for use in medullary thyroid cancer and as a second-line agent in advanced renal cell carcinoma. In vitro studies found it to exhibit excellent activity against both the wild-type ROS1 fusion and the G2032R and G2026M mutations at concentrations less than 30?nmol/L—a dose much lower than what is clinically achievable [71, 91]. It has been found to inhibit CD74-ROS1-transformed Ba/F3 cells with more potency than entrectinib, brigatinib, lorlatinib [92], or foretinib [71].
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased by
clarithromycin and erythromycin; concentration
reduced by rifampicin - avoid.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone - avoid.
Antipsychotics: avoid with clozapine - increased risk
of agranulocytosis.
Metabolism
Cabozantinib is metabolised mostly by CYP3A4 and, to a minor extent, by CYP2C9. Both enzymes produce an N-oxide metabolite. Cabozantinib is eliminated mainly by the faeces (54%) and also by the urine (27%).
storage
Store at +4°C
References
1) Yakes?et al.?(2011),?Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth; Mol. Cancer Ther.,?10?2298 2) You?et al.?(2011),?VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer; Cancer Res.,?71?4758 3) Kurzrock?et al.?(2011),?Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor in patients with medullary thyroid cancer; J. Clin. Oncol.,?29?2660
Cabozantinib Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jewim Pharmaceutical (Shandong) Co., Ltd. | +86-15552509998 +86-15621883869 | liutf@jewim.com.cn | China | 251 | 58 |
| Shanghai Joiny Pharmaceutical Co.,LTD | +8613681955282 | gyd@joiny-pharma.com | China | 228 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Nanjing Gold Pharmaceutical Technology Co. Ltd. | 025-84209270 15906146951 | CHINA | 115 | 55 | |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
Related articles
- Cabozantinib:Efficacy,effectiveness and safety
- Cabozantinib effectively improves survival in renal cell carcinoma and hepatocellular carcinoma, offering benefits over altern....
- Feb 7,2024
- Cabozantinib: mechanism of action, pharmacokinetics and clinical applications
- Cabozantinib is a multi-targeted tyrosine kinase inhibitor used for thyroid, renal, and liver cancer. It enhances the immune s....
- Jul 27,2023
- Cabozantinib (XL184)- antitumour effects
- Cabozantinib is a small molecule inhibitor of multiple tyrosine kinase receptors. In vitro, it has demonstrated inhibitory act....
- Dec 13,2019
View Lastest Price from Cabozantinib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
![N-[4-[(6,7-Dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide pictures](/ProductImageEN/2023-09/Small/b566bf0d-fda0-4dff-ba87-e69f67574627.png) |
2024-04-10 | N-[4-[(6,7-Dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide
849217-68-1
|
US $0.00 / kg | 1kg | 98% | 500kg | Shanghai Joiny Pharmaceutical Co.,LTD | |
 |
2024-03-12 | Cabozantinib
849217-68-1
|
US $0.00 / g | 1g | 98% HPLC | 1kg | shandong perfect biotechnology co.ltd | |
 |
2023-12-26 | Cabozantinib
849217-68-1
|
US $0.00-0.00 / kg | 1kg | 99%, Single impurity<0.1 | 1 ton | Nanjing Fred Technology Co., Ltd |
-
![N-[4-[(6,7-Dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide pictures](/ProductImageEN/2023-09/Small/b566bf0d-fda0-4dff-ba87-e69f67574627.png)
- N-[4-[(6,7-Dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide
849217-68-1
- US $0.00 / kg
- 98%
- Shanghai Joiny Pharmaceutical Co.,LTD
-

- Cabozantinib
849217-68-1
- US $0.00 / g
- 98% HPLC
- shandong perfect biotechnology co.ltd
-

- Cabozantinib
849217-68-1
- US $0.00-0.00 / kg
- 99%, Single impurity<0.1
- Nanjing Fred Technology Co., Ltd