(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
- CAS No.
- 1268524-70-4
- Chemical Name:
- (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
- Synonyms
- tert-butyl (S)-2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate;(+)-JQ1;(+)-JQ-1;jq01;S-JQ1;CS-799;(+)JQ1;JQ1 compound;(+)-JQ1, >=99%;3,9-trimethyl-6
- CBNumber:
- CB72572988
- Molecular Formula:
- C23H25ClN4O2S
- Molecular Weight:
- 456.99
- MDL Number:
- MFCD22683748
- MOL File:
- 1268524-70-4.mol
- MSDS File:
- SDS
(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate Properties
| Melting point | >205°C (dec.) |
|---|---|
| Boiling point | 610.4±65.0 °C(Predicted) |
| Density | 1.33 |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| pka | 2.05±0.60(Predicted) |
| color | white to beige |
| Stability | Stable for 1 year as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
| InChIKey | DNVXATUJJDPFDM-KRWDZBQOSA-N |
| FDA UNII | 1MRH0IMX0W |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H332-H335 |
| Precautionary statements | P261-P280-P305+P351+P338 |
(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate price More Price(44)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML1524 | (+)-JQ1 ≥98% (HPLC) | 1268524-70-4 | 5mg | $196 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML1524 | (+)-JQ1 ≥98% (HPLC) | 1268524-70-4 | 25mg | $711 | 2024-03-01 | Buy |
| Cayman Chemical | 11187 | (+)-JQ1 ≥98% | 1268524-70-4 | 1mg | $28 | 2024-03-01 | Buy |
| Cayman Chemical | 11187 | (+)-JQ1 ≥98% | 1268524-70-4 | 5mg | $98 | 2024-03-01 | Buy |
| Cayman Chemical | 11187 | (+)-JQ1 ≥98% | 1268524-70-4 | 10mg | $140 | 2024-03-01 | Buy |
(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate Chemical Properties,Uses,Production
Biological Activity
(+)-JQ1 is a BET bromodomain inhibitor that acts on BRD4 (2) with C50 being 33nM. Except the BET family, (+)-JQ1 does not bind to the Bromodomain structure domain of other family.
In vitro
(+)-JQ1 enantiomer can bind to directly the Kac binding site of BET bromodomain structure domain. (+)-JQ1 (500nM) and chromatin competitively bind to BRD4, leading to NMC cell differentiation and growth standstill. The Ki67 staining decrease proves that (+)-JQ1(500nM) weakens the rapid proliferation of NMC 797 and Per403 cell system. (+)-JQ1(500nM) acts on NMC 797 cells, effectively reducing the expression of BRD4 target gene. (+)-JQ1acts on NMC11060 cells inhibiting cell activity with IC50 being 4nM. (+)-JQ1 acts on MM cell system and strongly inhibits MYC expression. (+)-JQ1 inhibits the proliferation of KMS-34 and LR5 with IC50 being 68nM and 98nM respectively. After being treated by (+)-JQ1(500nM), the proportion of MM.1S cell in S phase decrease and more cells stasis in the G0/G1 phase.
(+)-JQ1(500nM) dyed by β-galactosidase results in significant cell senescence. In the CD138+-derived MM sample treated by (+)-JQ1(800nM), the cell activity significantly decrease. (+)-JQ1 inhibits LP-1 cell growth with GI50 being 98nM. (+)-JQ1 (625nM) resulted in an increase in the proportion of LP-1 cells at G0/G1 phase. (+)-JQ1 (500nM) acts on LP-1 cells inhibiting MYC, BRD4 and CDK9 expression. (+)-JQ1 (1 μM) deals with latent infection of Jurkat T cells and activates HIV transcription. (+)-JQ1 (50μM) acts on Jurkat and HeLa cells, primarily stimulating Tat-dependent HIV transcription. (+)-JQ1 (5 μM) acts on J-Lat A2 cell inducing Brd4 dissociation, allowing Tat to recruit SEC to the HIV promoter and inducing Pol II CTD phosphorylation and viral transcription.
In vivo
(+)-JQ1(50mg /kg) treats mice with NMC797 transplanted tumors inhibiting tumor growth. (+)-JQ1(50mg/kg) erases NUT nuclear spots in mice with NMC797 transplanted tumors, consistent with competitive binding to nuclear chromatin. (+)-JQ1 (50mg/kg) treats NMC797 transplanted tumor, significantly inducing (31grade) keratin expression. (+)-JQ1(50mg/kg) treats mouse model carrying NMC transplanted tumor, promoting differentiation, tumor decline and prolonging life. (+)-JQ1(50mg/kg) treats MM.1S-luc+ cells through intravenous injection. Compared with animals in control group, the life of SCID beige mice carrying orthotopic transplanted tumors significantly extends. Through intraperitoneal injection (+)-JQ1(50mg/kg) can prolong the life of mice carrying Raji xenograft greatly.
Characteristic
(+)-JQ1 is more effective than (-)-JQ1.
Description
JQ1 (+) (1268524-70-4) is a potent BET bromodomain inhibitor and is the active isomer.?? IC50 = 17.7, 32.6, 76.9 and 12942 nM respectively for BRD2 (N-terminal (N)), BRD4 (C-terminal (C)), BRD4 (N) and CREBBP respectively (data for + isomer).1 Competitive binding by JQ1 displaces the BRD4 fusion oncoprotein from chromatin, prompting squamous differentiation and specific antiproliferative effects in BRD4-dependent cell lines and patient-derived xenograft models.1?Induces squamous differentiation in NMC cell lines and inhibits tumor growth in NMC xenografts.2?Displays reversible contraceptive effects in male mice.3?Blocks inflammation and bone loss in periodontitis.4?Reverses CAR T cell extinction.5
Uses
(+)-JQ1 has been used in flow cytometry assay, cell viability assay and quantitative PCR assay in order to investigate on the reversal of HIV-1 latency.
Uses
BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation.
Definition
ChEBI: JQ1 is a member of the class of thienotriazolodiazepines that is the tert-butyl ester of [(6S)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl]acetic acid. An inhibitor of bromodomain-containing protein 4 that exhibits anti-cancer and cardioprotective properties. It has a role as a bromodomain-containing protein 4 inhibitor, a cardioprotective agent, an antineoplastic agent, an anti-inflammatory agent, an angiogenesis inhibitor, an apoptosis inducer and a ferroptosis inducer. It is a thienotriazolodiazepine, an organochlorine compound, a carboxylic ester and a tert-butyl ester.
General Description
JQ1 is a member of the triazolo-diazepine compound family, which functions as a pan-BET (bromodomain and extra-terminal motif) family inhibitor. JQ1 is known to suppress cell proliferation and therefore, can be used as a therapeutic drug for a number of cancers including multiple myeloma and acute myeloid leukemia.
Biochem/physiol Actions
(+)-JQ1 is a high affinity, potent and selective inhibitor of BET bromodomain proteins, including BRD2, BRD3, BRD4 and BRDT. (+)-JQ1 (also known as SGCBD01), the active enantiomer of (+/-)-JQ1, inhibits Brd4 (bromodomain-containing 4), which forms complexes with chromatin via two tandem bromodomains (BD1 and BD2) that bind to acetylated lysine residues in histones and Brd4 association with acetylated chromatin is believed to regulate the recruitment of elongation factor b and additional transcription factors to specific promoter regions. The nuclear protein in testis (NUT) gene is known to form fusions with Brd4 that create a potent oncogene, leading to rare, but highly lethal tumors referred to as NUT midline carcinomas (NMC). (+)-JQ1 inhibits recruitment and binding of Brd4 to TNFa and E-selectin promoter elements, and accelerates recovery time in FRAP (fluorescence recovery after photobleaching) assays using GFP-Brd4. Thus (+)-JQ1 is a useful tool to study the role of Brd4 in transcriptional initiation.For characterization details of (+)-JQ1, please visit the JQ-1 probe summary on the Structural Genomics Consortium (SGC) website.(-)-JQ1 is the negative control for the active enantiomer, (+)-JQ1. (-)-JQ1 is available from Sigma. To learn more about and purchase (-)-JQ1, click here.To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
storage
-20°C
References
References/Citations:
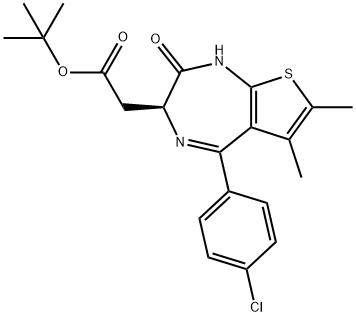
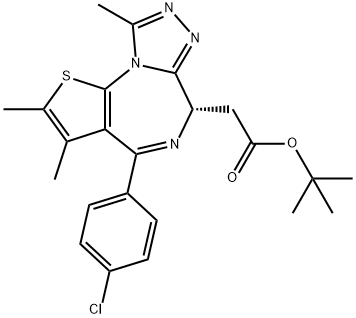
(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | 18957127338 | stella@zetchem.com | China | 2141 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| Jilin Chinese Academy of Sciences - Yanshen Technology Co., Ltd. | 0431-80514535 13634302652 | Extension@chemextension.com | CHINA | 967 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
Related articles
- (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-t - Reaction / Application on synthetic works
- JQ1 is a thienotriazolodiazepine and a potent inhibitor of the BET (bromodomain and extra-terminal motif) family of bromodomai....
- Nov 19,2019
View Lastest Price from (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate pictures](https://img.chemicalbook.com/ProductImageEN/2020-3/Small/cfa6bbcf-975a-4c2c-9364-ceeaab9583cd.jpg) |
2020-03-06 | (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
12688524-70-4
|
US $1.00 / KG | 1KG | 97%-99.9% | 200kg | Career Henan Chemical Co | |
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate pictures](https://img.chemicalbook.com/ProductImageEN/2020-1/Small/6dce4ff0-6df7-49e7-aaa6-e88d8213cc93.jpg) |
2020-01-14 | (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
1268524-70-4
|
US $7.00 / KG | 1KG | 99% | 100KG | Career Henan Chemical Co | |
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate pictures](https://img.chemicalbook.com/ProductImageEN/2018-8/Small/63316eb0-d554-4853-9d8b-77af49db8342.gif) |
2019-07-06 | (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
1268524-70-4
|
US $1.00 / KG | 1G | 98% | 100KG | Career Henan Chemical Co |
-
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate pictures](https://img.chemicalbook.com/ProductImageEN/2020-3/Small/cfa6bbcf-975a-4c2c-9364-ceeaab9583cd.jpg)
- (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
12688524-70-4
- US $1.00 / KG
- 97%-99.9%
- Career Henan Chemical Co
-
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate pictures](https://img.chemicalbook.com/ProductImageEN/2020-1/Small/6dce4ff0-6df7-49e7-aaa6-e88d8213cc93.jpg)
- (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
1268524-70-4
- US $7.00 / KG
- 99%
- Career Henan Chemical Co
-
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate pictures](https://img.chemicalbook.com/ProductImageEN/2018-8/Small/63316eb0-d554-4853-9d8b-77af49db8342.gif)
- (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
1268524-70-4
- US $1.00 / KG
- 98%
- Career Henan Chemical Co





![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate Structure](CAS/GIF/1268524-70-4.gif)