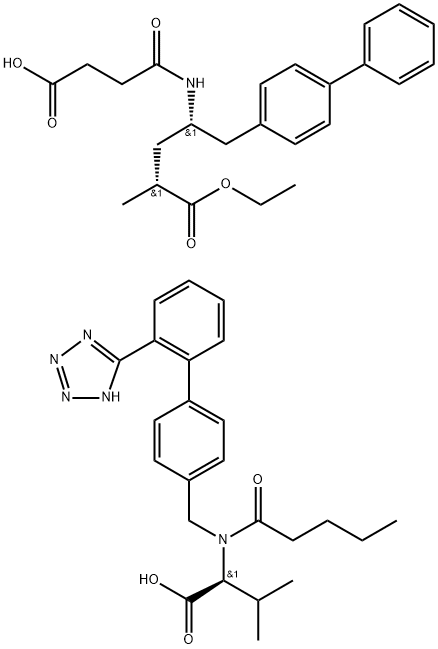
LCZ696
- CAS No.
- 936623-90-4
- Chemical Name:
- LCZ696
- Synonyms
- Entresto;LZC696;Valsartan/sacubitril;LCZ696(Sacubitril/Valsartan);Sacubitril/Valsartan Trisodium Salt;Sacubitril valsartan sodium hydrate;LCZ696;LCA696;Secubitril;LCZ696;LCZ 696
- CBNumber:
- CB72715242
- Molecular Formula:
- C48H58N6O8
- Molecular Weight:
- 847.03
- MDL Number:
- MFCD29477717
- MOL File:
- 936623-90-4.mol
- MSDS File:
- SDS
| storage temp. | Store at -20°C |
|---|---|
| solubility | ≥45.05 mg/mL in DMSO; ≥11.28 mg/mL in H2O; ≥28.5 mg/mL in EtOH |
| form | solid |
| InChIKey | XTKIDERFOUYBDE-NOFROVMTNA-N |
| SMILES | C(C1C=CC(C2C=CC=CC=2C2=NN=NN2)=CC=1)N(C(=O)CCCC)[C@H](C(=O)O)C(C)C.C(C1C=CC(C2C=CC=CC=2)=CC=1)[C@@H](NC(=O)CCC(=O)O)C[C@@H](C)C(=O)OCC |&1:25,45,55,r| |
| FDA UNII | WB8FT61183 |
| NCI Drug Dictionary | Entresto |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302+H312-H315+H320 |
LCZ696 price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 23425 | LCZ696 | 936623-90-4 | 10mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 23425 | LCZ696 | 936623-90-4 | 25mg | $72 | 2024-03-01 | Buy |
| Cayman Chemical | 23425 | LCZ696 | 936623-90-4 | 50mg | $116 | 2024-03-01 | Buy |
| TRC | L270005 | LCZ696 | 936623-90-4 | 25mg | $75 | 2021-12-16 | Buy |
| TRC | L270005 | LCZ696 | 936623-90-4 | 5mg | $50 | 2021-12-16 | Buy |
LCZ696 Chemical Properties,Uses,Production
Drugs for heart failure
LCZ696 a drug developed by Novartis Pharmaceutical of the United States for the treatment of heart failure. Heart failure is a life-threatening disease. The patient's heart cannot pump enough blood to supply the body, and appear the symptoms of dyspnea, fatigue and fluid retention slowly, then gradually increased, significantly affect the quality of life.
LCZ696 is a dual-acting angiotensin-receptor enkephalinase inhibitor that has a unique mechanism that is thought to reduce the strain of failing hearts. LCZ696 can enhance the body's natural defense against heart failure, as well as increase the level of natriuretic peptides and other endogenous vasoactive peptides, and inhibit the renin-angiotensin-aldosterone system (RAAS). LCZ696 has combined with Novartis's Hypertension Medication (Diovan, generic name: valsartan) and the experimental drug AHU-377. AHU377 blocks the mechanism of the two peptides responsible for lowering blood pressure. Diovan improves vasodilatation and stimulates the body to excrete sodium and water.
The safety threshold of cardiovascular drugs is extremely high, and LCZ696 even shows a higher safety than conventional drugs. Previously, Novartis vigorously develop the cardiovascular drug— serelaxi, while because of security issues, both the FDA and the European Union did not grant this drug. This is undoubtedly a heavy blow to Novartis. However, the success of LCZ696 will pay back Novartis a big era in the cardiovascular field.。
In August 2014, Novartis announced significant results as a landmark of PARADIGM-HF trial. LCZ696 was significantly superior to the ACE inhibitor Enalapril at several key end points, including a significant reduction in cardiovascular death risk or heart failure hospitalization rate. These latest analyzes were first disclosed at the American Heart Association Scientific Conference in 2014 and published in Circulation at the same time.
On November 30, 2014, the European Medicines Agency (EMP) Committee for Medicinal Products for Human Use (CHMP) has granted LCZ696 an accelerated eligibility, so it became the first cardiovascular drug to qualify for accelerated evaluation in the history of EU drug regulation. Prior to this, the EU's Accelerated Assessment of Qualifications was never granted to the cardiovascular field.
LCZ696 is an experimental drug developed for the treatment of patients with Heart Failure with reduced Ejection Fraction (HFrEF). Accreditation of Accelerated Evaluation by CHMP means that the review time of LCZ696 will be shortened by 60 days in official EU. Novartis is expected to submit LCZ696 application approval (MAA) in the European Union at the beginning of 2015. The MAA submission will be based on data from the landmark Phase III PARADIGM-HF study, which is the largest study of heart failure patients conducted in the history. The data show that the efficacy and safety of LCZ696 is beyond the clinical standard drug enalapril (enalapril), including significantly reduced cardiovascular death or heart failure hospitalization risk
The industry believes that LCZ696's outstanding performance, make it became one of the most important progress over the past 10 years in the field of cardiology. At the same time, in the next few years, there will be no drug to compete with the LCZ696 in the cardiovascular field. Some analysts predict that the LCZ696 sales peaked at $ 8 billion; while Deutsche Bank analysts expect the drug to peak at $ 6 billion, given LCZ696's superior performance in reducing cardiovascular risk. Although the data are slightly different, but there is no doubt that, LCZ696 will become a super star for Novartis. In addition to bring rolling financial resources, it will lead the cardiovascular treatment step into a new era.
In February 2015, Novartis Pharma Test Drug LCZ696 was given priority review by the US Food and Drug Administration (FDA) for the treatment of Heart Failure with reduced Ejection Fraction (HFrEF). Novartis added that the drug's overall review time will be reduced by 8 months, the FDA may make its approval decision in August.
Novartis said its listing application in US was based on a 3-phase PARADIGM-HF study, which showed a significant reduction in cardiovascular mortality and heart failure hospitalization compared with the ACE inhibitor Enalapril.
This information is compiled and edited by Nan, Xiao Nan from ChemicalBook.
bioactivity
LCZ696, composed of valsartan and sacubitril in a 1: 1 molar ratio, is an orally bioavailable, dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) for the treatment of hypertension and heart failure.
In vivo studies
In a double transgenic, overexpressed human renin, angiotensinogen and plasma atrial natriuretic peptide immunoreactivity rat, LCZ696 (60 mg/kg p.o.) induced a persistent decrease and dose-dependence in mean arterial pressure (MAP) and , and stimulated dose-dependent rapid increase of ANP immunoreactivity in plasma. LCZ696 (68 mg/kg p.o.) attenuates cardiac remodeling and dysfunction by reducing myocardial fibrosis and cardiac hypertrophy in a rat myocardial infarction (MI) model.
Description
LCZ696 is a dual angiotensin II receptor antagonist and neprilysin inhibitor that is a combination of the nonpeptide angiotensin II receptor antagonist valsartan and AHU377 , a prodrug of LBQ657 , which is an inhibitor of the zinc metallopeptidase neprilysin., LCZ696 (2-60 mg/kg) induces a dose-dependent decrease in mean arterial pressure in rats expressing human renin and angiotensinogen, a double-transgenic model for angiotensin II-dependent hypertension. Formulations containing LCZ696 are under clinical investigation for the treatment of mild to moderate hypertension and chronic heart failure.
Mechanism of action
LCZ696 is a molecule that combines the moieties of valsartan and sacubitril (the neprilysin inhibitor) in a single substance. By inhibiting neprilysin, the degradation of natriuretic peptides, bradykinin, and other peptides is slowed. Hence, high circulating levels of natriuretic peptides enhance diuresis , natriuresis, myocardial relaxation, and anti-remodelling, on top of the ARB effects of valsartan.
Clinical Use
LCZ696 (Novartis Pharmaceuticals), a first-in-class inhibitor of dualacting angiotensin-2 type-I receptor and neprilysin inhibitors, was developed as a molecule composed of molecular moieties of valsartan and neprilysin inhibitor prodrug AHU377 in a 1:1 ratio. Several phase 2 randomised clinical trials have been done or are underway in patients with hypertension. In a proof-of-concept randomised trial, LCZ696 was compared with valsartan in 1328 patients with mild-to-moderate hypertension, and provided fully additive reduction of blood pressure. No cases of angio-oedema were reported in the 8 week treatment period. This tolerance should be confirmed, particularly in black patients, for whom omapatrilat resulted in a higher occurrence of angio-oedema than in white patients. Indeed, in the proof-of-concept trial only about 8% of patients were black.
References
1. McMurray, John J. V. et al. “Angiotensin-neprilysin inhibition versus enalapril in heart failure.” The New England journal of medicine 371 11 (2014): 993-1004. DOI: 10.1056/NEJMoa1409077
2. Gu, Jessie et al. “Pharmacokinetics and Pharmacodynamics of LCZ696, a Novel Dual‐Acting Angiotensin Receptor—Neprilysin Inhibitor (ARNi).” The
Journal of Clinical Pharmacology 50 (2010): n. pag. DOI: 10.1177/0091270009343932
3. Langenickel, T.H., & Dole, W.P. (2012). Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure. Drug Discovery Today: Therapeutic Strategies, 9. DOI: 10.1016/J.DDSTR.2013.11.002
4. Suematsu, Yasunori et al. “LCZ696, an angiotensin receptor–neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin‐induced diabetic mice.” European Journal of Heart Failure 18 (2016): n. pag. DOI: 10.1002/ejhf.474
LCZ696 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zibo Wei Bin Import & Export Trade Co. Ltd. | +86-0533-2091136 +8613864437655 | ziboweibinmaoyi@163.com | China | 100 | 58 |
| ChemExpress | +86-021-58950125 | info@chemexpress.com | China | 557 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7613 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 40 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 1175 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Nanjing Gold Pharmaceutical Technology Co. Ltd. | 025-84209270 15906146951 | CHINA | 115 | 55 | |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
View Lastest Price from LCZ696 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Sacubitril valsartan
936623-90-4
|
US $50.00 / kg | 1kg | 99.9% | 200000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-22 | Sacubitril Valsartan sodium
936623-90-4
|
US $0.00-0.00 / Kg/Bag | 1Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-10 | LCZ696
936623-90-4
|
US $0.00-0.00 / KG | 1KG | 99% | 1000KG | Hangzhou Hyper Chemicals Limited |
-

- Sacubitril valsartan
936623-90-4
- US $50.00 / kg
- 99.9%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- Sacubitril Valsartan sodium
936623-90-4
- US $0.00-0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- LCZ696
936623-90-4
- US $0.00-0.00 / KG
- 99%
- Hangzhou Hyper Chemicals Limited
936623-90-4(LCZ696)Related Search:
1of4