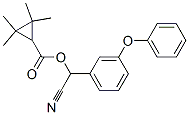
Fenpropathrin
- CAS No.
- 64257-84-7
- Chemical Name:
- Fenpropathrin
- Synonyms
- 2,2,3,3-tetramethylcyclopropanecarboxylicacidcyano(3-phenoxyphenyl)methylest;RODY;HERALD;AMITOL;PLATINO;DIGITAL;MEOTHRIN;FENOTHRIN;Hsdb 6636;Fenpropathrin
- CBNumber:
- CB7349975
- Molecular Formula:
- C22H23NO3
- Molecular Weight:
- 349.42
- MDL Number:
- MFCD00144305
- MOL File:
- 64257-84-7.mol
| Melting point | 45-50°C |
|---|---|
| Boiling point | 483.6°C (rough estimate) |
| Density | 1.1500 |
| vapor pressure | 7.3×10-4 Pa (20 °C) |
| refractive index | 1.5614 (estimate) |
| storage temp. | 0-6°C |
| Water Solubility | 0.014 mg l-1 (25 °C) |
| CAS DataBase Reference | 64257-84-7(CAS DataBase Reference) |
| FDA UNII | 87BH96P0MX |
| EPA Substance Registry System | Cyclopropanecarboxylic acid, 2,2,3,3-tetramethyl-, cyano(3-phenoxyphenyl)methyl ester(64257-84-7) |
Fenpropathrin Chemical Properties,Uses,Production
Uses
Fenpropathrin controls many species of mites and insects on pome fruit, citrus, vines, hops, vegetables, cotton, ornamentals and glasshouse crops such as tomatoes.
Definition
ChEBI: Fenpropathrin is a cyclopropanecarboxylate ester obtained by formal condensation between 2,2,3,3-tetramethylcyclopropanecarboxylic acid and cyano(3-phenoxyphenyl)methanol. It has a role as a pyrethroid ester insecticide, a pyrethroid ester acaricide and an agrochemical. It is an aromatic ether and a cyclopropanecarboxylate ester. It is functionally related to a 2,2,3,3-tetramethylcyclopropanecarboxylic acid.
Metabolic pathway
Fenpropathrin possesses only one chiral centre (at benzylic carbon) and therefore presents a much simpler stereochemical picture than that seen with most of the other pyrethroids. Most metabolic work has been conducted with the RS racemate. Solution and surface photochemistry, and degradation in water, soils, plants and animals, have been reported. The fate of the 3-phenoxybenzyl portion of the molecule is very similar to that reported for cypermethrin and other analogues. Degradation is mainly by ester cleavage and hydroxylation.
Degradation
A detailed study of the kinetics of the hydrolysis of [14C-cyclopropyl]-
fenpropathrin and [14C-benzyl]fenpropathrins howed that ester bond
cleavage predominated over cyan0 group hydration (Takahashi et al.,
1985a). Hydrolysis in a series of buffers gave the following DT50 values at
25 °C: pH 7, >2 years; pH 9, 8 days; pH 10, <1 day. A base-catalysed
process operates above pH 7. Products detected were 2,2,3,3-tetramethylcyclopropanecarboxylic
acid (TMCA, 2), 3PBAl(3) and the amide
(4) (Scheme 1).
Fenpropathrin (labelled as above) was subject to slow photodegradation
in sunlight under various conditions with the following initial halflives:
distilled water, >6 weeks; humic acid solution, 6 weeks; river water,
2.7 weeks; sea water, 1.6 weeks; 2% aqueous acetone, 0.5 day. Half-lives
on three soils ranged from 1 to 5 days and on mandarin orange leaves it was
6 days (Takahashi et al., 1985b). The major products were TMCA, the
amide (4) and 2-(3-phenoxybenzyl)-2-(2,2,3,3-tetraethylcyclopropyl)-
acetonitrile (5). The latter product appeared to be unique to aqueous
photolysis. By far the major product found on soil surfaces was the amide
(4) but this was also found under dark conditions and is mainly a thermal
product.
Many other minor products were detected indicating the occurrence of
(i) hydroxylation at a methyl group, (ii) oxidation to 3PBA (7), (iii)
0-dephenylation to afford 3-hydroxybenzoic acid (8) and (iv) loss of CN
as CO2,. After 14 days, about 50% of the applied radioactivity was bound
to a high organic matter soil. This was photochemically-induced, as less
than 2% was bound in the dark. More recent studies using a xenon lamp
(Katagi, 1993) indicated that formation of the amide was most efficient
under drier conditions. Increased moisture, particularly in soil containing
acidic binding sites, favoured ester cleavage.
Degradation in organic solvents and in thin films afforded similar
results (Dureja, 1989). The pathways of photodegradation of fenpropathrin
are illustrated in Scheme 1.
Fenpropathrin Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
64257-84-7(Fenpropathrin)Related Search:
1of4