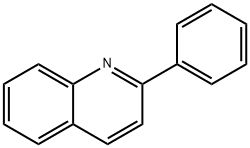
2-Phenylquinoline
- CAS No.
- 612-96-4
- Chemical Name:
- 2-Phenylquinoline
- Synonyms
- 830g;2-Phenylquinoline;a-Phenylquinoline;Phenylquinoline,99%;2-Phenylquinoline>Phenylquinoline, 99%;Quinoline, 2-phenyl-;2-Phenylquinoline 99%;alpha-Phenylquinoline;2-Phenylquinoline, 99+%
- CBNumber:
- CB7408748
- Molecular Formula:
- C15H11N
- Molecular Weight:
- 205.25
- MDL Number:
- MFCD00011568
- MOL File:
- 612-96-4.mol
- MSDS File:
- SDS
| Melting point | 84-85 °C (lit.) |
|---|---|
| Boiling point | 363 °C |
| Density | 1.1155 (rough estimate) |
| refractive index | 1.6550 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| pka | 4.52±0.10(Predicted) |
| form | Crystalline Powder |
| color | White to light yellow |
| Water Solubility | SLIGHTLY SOLUBLE |
| λmax | 258nm(lit.) |
| CAS DataBase Reference | 612-96-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Quinoline, 2-phenyl-(612-96-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29334900 | |||||||||
| NFPA 704 |
|
2-Phenylquinoline price More Price(34)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 299650 | 2-Phenylquinoline 99% | 612-96-4 | 1g | $80.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 299650 | 2-Phenylquinoline 99% | 612-96-4 | 5g | $384 | 2024-03-01 | Buy |
| TCI Chemical | P2057 | 2-Phenylquinoline >98.0%(GC)(T) | 612-96-4 | 1g | $66 | 2024-03-01 | Buy |
| TCI Chemical | P2057 | 2-Phenylquinoline >98.0%(GC)(T) | 612-96-4 | 5g | $223 | 2024-03-01 | Buy |
| Alfa Aesar | H31920 | 2-Phenylquinoline, 99+% | 612-96-4 | 1g | $70.65 | 2024-03-01 | Buy |
2-Phenylquinoline Chemical Properties,Uses,Production
Description
2-Phenylquinoline is the major quinoline alkaloid of Galipea iongiflora, a Bolivian plant used as treatment for cutaneous leishmaniasis. Antinociceptive properties of 2-phenylquinoline isolated from the bark of Galipea iongiflora against different models of pain in mice were evaluated.
Chemical Properties
white to light yellow crystalline powder
Uses
2-Phenylquinoline was used in quantitative structure-activity relationship (QSAR) analyses of estrogen receptor β-selective ligands.
Preparation
Synthesis of 2-phenylquinoline: Quinoline (1.0 g, 7.742 mmol) and phenyl lithium (2.30 mL, 2 M, 23.22 mmol) were reacted according to general procedure. Purification of the residue by silica gel column chromatography (EtOAc:MeOH:Et3N; 10-30:1:1 or PhMe:MeOH:Et3N; 10:1:1) gave 2-phenylquinoline (0.66 g, 42%) as an orange solid.
Aniline (0.140 g, 1.50 mmol) and cinnamaldehyde (0.132 g, 1.00 mmol) were dissolved in toluene in a reaction vial equipped with a magnetic stirrer bar, followed by the addition of K10 (0.50 g). The reaction mixture was heated at a temperature of 110 ?C for 3 hours. After completion of the reaction, the crude product was purified by column chromatography over silica gel eluting with a mixture of Hexane : Ethyl acetate (20:1) to produce 2-Phenylquinoline as a yellow solid (0.044 g, 21%); (m.p. 82-84 ?C) (lit. 84-85 °C); Rf 0.67 (20:1 hexane:ethyl acetate);
1H NMR (400 MHz, CDCl3) δH 7.46-7.51 (1H, m, H-4’), 7.53-7.56 (3H, m, H-6, 3’, 5’), 7.73- 7.77 (1H, m, H-7), 7.85 (1H, d, J = 8.31 Hz, H-5), 7.88-7.91 (1H, d, J = 8.31 Hz, H-3), 8.18- 8.27 (4H, m, H-4, 8, 2’, 6’)
13C NMR(400 MHz, CDCl3) δC 119.2 (C-3), 126.7 (C-6), 127.2 (C-4a), 127.5 (C-2’, 6’), 127.9 (C-5), 128.4 (C-3’, 5’), 128.7 (C-4’), 128.9 (C-7, 8), 129.8 (C-4), 130.3 (C-1’), 137.9 (C-8a), 157.2 (C-2)
Synthesis Reference(s)
Synthetic Communications, 23, p. 1959, 1993 DOI: 10.1080/00397919308009854
Chemical and Pharmaceutical Bulletin, 26, p. 3485, 1978 DOI: 10.1248/cpb.26.3485
Journal of the American Chemical Society, 71, p. 2327, 1949 DOI: 10.1021/ja01175a017
2-Phenylquinoline Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10248 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81148696 +8615536356810 | 1047@dideu.com | China | 3459 | 58 |
View Lastest Price from 2-Phenylquinoline manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-01-06 | 2-Phenylquinoline
612-96-4
|
US $188.00-1.00 / KG | 1KG | 99%, 99.5% Sublimated | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2022-03-07 | 2-Phenylquinoline
612-96-4
|
US $1.10 / g | 1g | 99.00% | 100 Tons | Dideu Industries Group Limited | |
 |
2020-04-30 | 2-Phenylquinoline
612-96-4
|
US $0.10 / KG | 1KG | 99.0% | 1000 tons | Shaanxi Dideu Medichem Co. Ltd |
-

- 2-Phenylquinoline
612-96-4
- US $188.00-1.00 / KG
- 99%, 99.5% Sublimated
- Henan Fengda Chemical Co., Ltd
-

- 2-Phenylquinoline
612-96-4
- US $1.10 / g
- 99.00%
- Dideu Industries Group Limited
-

- 2-Phenylquinoline
612-96-4
- US $0.10 / KG
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd
612-96-4(2-Phenylquinoline)Related Search:
1of4