Tamibarotene
- CAS No.
- 94497-51-5
- Chemical Name:
- Tamibarotene
- Synonyms
- AM80;C12864;Amnoid;CS-1151;Amnolake;NSC 608000;amibarotene;Tamibarotene;UNII-08V52GZ;Retinoid AM 80
- CBNumber:
- CB7426841
- Molecular Formula:
- C22H25NO3
- Molecular Weight:
- 351.44
- MDL Number:
- MFCD00866188
- MOL File:
- 94497-51-5.mol
- MSDS File:
- SDS
| Melting point | 231-232°C |
|---|---|
| Boiling point | 449.6±45.0 °C(Predicted) |
| Density | 1.154±0.06 g/cm3(Predicted) |
| RTECS | DH6940000 |
| storage temp. | room temp |
| solubility | Soluble to 50 mM in DMSO |
| pka | 3.83±0.10(Predicted) |
| form | powder |
| color | white to off-white |
| FDA UNII | 08V52GZ3H9 |
| NCI Drug Dictionary | tamibarotene |
Tamibarotene price More Price(35)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | T3205 | Tamibarotene ≥98% (HPLC) | 94497-51-5 | 5mg | $101 | 2024-03-01 | Buy |
| Sigma-Aldrich | T3205 | Tamibarotene ≥98% (HPLC) | 94497-51-5 | 25mg | $393 | 2024-03-01 | Buy |
| Alfa Aesar | J64028 | Tamibarotene | 94497-51-5 | 10mg | $157.65 | 2024-03-01 | Buy |
| Alfa Aesar | J64028 | Tamibarotene | 94497-51-5 | 50mg | $517.65 | 2024-03-01 | Buy |
| Cayman Chemical | 71770 | AM80 ≥98% | 94497-51-5 | 5mg | $81 | 2024-03-01 | Buy |
Tamibarotene Chemical Properties,Uses,Production
Description
Tamibarotene (brand name: Amnolake) is a kind of orally administrated synthetic retinoid. It is used to overcome the all- trans retinoid acid (ATRA) resistance, having potential antineoplastic activity against the acute promyelocytic leukaemia (APL). It can also be used for the treatment of Alzheimer's disease, multiple myeloma and Crohn's disease. Tamibarotene takes effect through directly acting as the specific agonist for retinoid acid receptor alpha/beta, and potentially binding to the retinoid X receptors (RXR). Compared to the ATRA, Tamibarotene is chemically more stable and much more potent as an inducer of differentiation and apoptosis in promyelocytic leukemia cells.
References
https://en.wikipedia.org/wiki/Tamibarotene
https://www.drugbank.ca/drugs/DB04942
Description
AM80 is a retinoic acid receptor agonist that is selective for RARα (Kd = 62 nM; AC50 = 1.46 nM) compared to RARβ (Kd = 280 nM; AC50 = 6.87 nM) and RARγ (Kd = 816 nM; AC50 = 148.7 nM). It demonstrates greater specific binding to RARα compared to retinoic acid , which exhibits little selectivity across RARα, β, or γ. AM80 exhibits antiproliferative effects against acute promyelocytic leukemia cells, inducing human promyelocytic leukemia HL-
Description
Tamibarotene, a selective agonist of the retinoic acid receptor, was launched in Japan as an oral treatment for relapsed or refractory acute promyelocytic leukemia (APL). APL is a form of acute myeloid leukemia (AML) characterized by a deficiency in mature blood cells and an excess of immature cells called promyelocytes in the bone marrow and peripheral blood. The current standard of care for APL includes treatment with all-trans-retinoic acid (ATRA), either alone or in combination with chemotherapy. ATRA is a high affinity ligand for two types of nuclear receptors, retinoic acid receptor (RAR) and retinoid X receptor (RXR), each of which has three subtypes (-α, -β, and -γ). Activation of RARα by ATRA causes promyelocytes to differentiate and mature, thereby inhibiting their proliferation and inducing disease remission. Although ATRA is one of the most clinically successful retinoids, its usage is hampered by the high rate of adverse effects, instability, and the appearance of ATRA-resistant leukemia cells.Adverse events included retinoic acid syndrome, hyperleukocytosis, xerosis, cheilitis, hypertriglyceridemia, and hypercholesterolemia; however, these side effects were generally milder than with ATRA, which all patients had received previously. Examination of human samples taken from Phase II and III clinical trials revealed that fecal excretion was the major elimination route, and the metabolism of tamibarotene occurred primarily through hydroxylation and taurine conjugation. In vitro, the plasma protein binding of tamibarotene is shown to be >98% in rats, dogs, and humans. Tamibarotene is synthesized from 5,5,8,8-tetramethyl-5,6,7, 8-tetrahydronaphthalene in a four-step sequence consisting of regioselective nitration in the 2-position, reduction of the nitro group by hydrogenation to produce the corresponding aniline derivative, acylation of the aniline intermediate with 4-(carbomethoxy)benzoyl chloride, and hydrolysis of the methyl ester.
Chemical Properties
Crystalline Solid
Originator
Toko Yakuhin Kogyo (Japan)
Uses
Tamibarotene, a retinoic acid receptor-α(RARα) agonist, was approved for the treatment of relapsed or refractory acute promyelocytic leukemia (APL) in Japan on June, 2005 and is currently marketed by Nippon Shinyaku Co. This novel drug has shown high remission rate among patients who have recurrent disease after all trans retinoic acid therapy.
Uses
A RARα agonist that induces differentiation and apoptosis
Uses
Synthetic retinoic acid receptor-α/β-selective retinoid. Antineoplastic.
Uses
Synthetic retinoic acid receptor-a/?selective retinoid. Antineoplastic
Definition
ChEBI: A dicarboxylic acid monoamide resulting from the condensation of one of the carboxy groups of terephthalic acid with the amino group of 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-amine.
brand name
Amnolake
Biological Activity
Retinoic acid receptor α (RAR α ) agonist that induces differentiation (ED 50 = 0.79 nM) and apoptosis of HL-60 cells in vitro . Exhibits antiproliferative effects against a variety of human tumor cells lines (mean values of 35, 40 and 60% growth inhibition at 0.1, 1 and 10 μ M respectively) and displays anticancer activity against acute promyelocytic leukemia in vivo .
Biochem/physiol Actions
Tamibarotene (Am80) is a RAR α agonist. Tamibarotene was developed to overcome resistance to ATRA and is currently approved in Japan for treatment of recurrent acute promyelocytic leukemia (APL). The compound induces HL-60 cells differentiation and apoptosis. Similarly to TTNPB, the compound neither binds to nor transactivates the RXRs. In contrast to TTNPB (pan RAR agonist), Tamibarotene is rather specific toward RAR α. The compound is approximate 10 times more potent than ATRA.
Synthesis
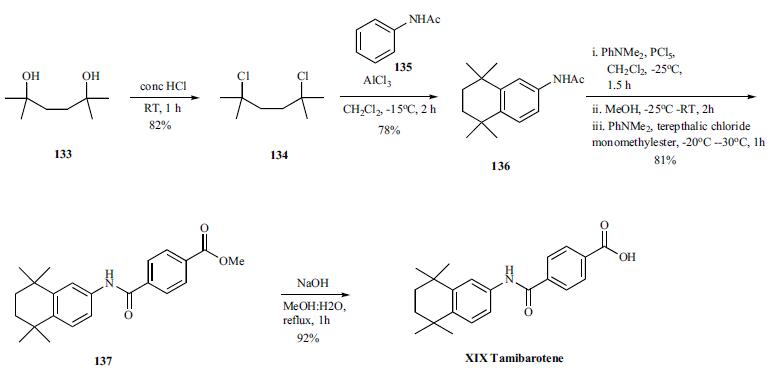
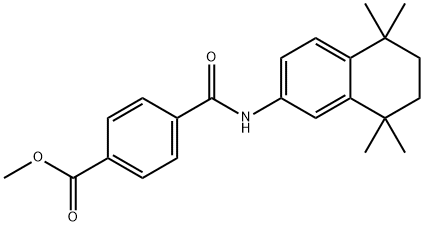
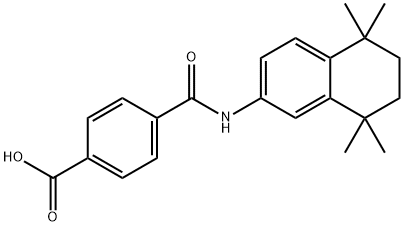
Several synthesis of tamibarotene have been disclosed in the literature including the process scale synthesis as shown in the scheme. The synthesis started with preparation of dichloride 134 in 82% yield from diol 133 by treating with concentrated HCL in DCM. Friedal Crafts reaction of dichloride 134 with acetanilide in the presence of aluminum chloride at -15??C for 2h provided acetanilide derivative 136 in 78% yield. In a single pot, the acetanilide was reacted with PCl5 and dimethylaniline at -25??C for 1.5h followed by quenching the reaction with methanol for 2h after addition at -25??C. Addition of dimethylaniline and terepthalic chloride mono-methylester at -30 - - 20??C for 1 hr provided the tamibarotene methyl easter 137 in 81% yield. Hydrolysis of the ester by heating with sodium hydroxide in MeOH:water mixture for 1h followed isolation and crystallization gave tamibarotene (XIX) in 92% yield.
storage
Store at RT
Tamibarotene Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shanghai Standard Technology Co., Ltd. | 18502101150 | ft-sales@nature-standard.com | CHINA | 1923 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| TopScience Biochemical | 00852-68527855 | info@itopbiochem.com | China Hong Kong | 902 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3632 | 58 |
View Lastest Price from Tamibarotene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-02-24 | Tamibarotene
94497-51-5
|
US $0.00 / mg | 20mg | ≥98%(HPLC) | 10 g | Shanghai Standard Technology Co., Ltd. | |
 |
2021-07-20 | Tamibarotene
94497-51-5
|
US $0.00-0.00 / KG | 5mg | 99% | 2000tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2020-03-06 | Tamibarotene
94497-51-5
|
US $1.00 / KG | 1KG | 99% | 200kgs | Career Henan Chemical Co |
-
- Tamibarotene
94497-51-5
- US $0.00 / mg
- ≥98%(HPLC)
- Shanghai Standard Technology Co., Ltd.
-

- Tamibarotene
94497-51-5
- US $0.00-0.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
-

- Tamibarotene
94497-51-5
- US $1.00 / KG
- 99%
- Career Henan Chemical Co
94497-51-5(Tamibarotene)Related Search:
1of4





