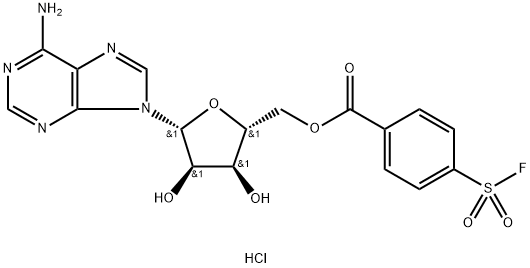
5'-P-FLUOROSULFONYL-BENZOYLADENOSINE HYDROCHLORIDE
- CAS No.
- 78859-42-4
- Chemical Name:
- 5'-P-FLUOROSULFONYL-BENZOYLADENOSINE HYDROCHLORIDE
- Synonyms
- FSBA;5'-FSBA;5'-Fluorosulfonylbenzoyladenosine;5'-p-Fluorosulfonylbenzoyl adenosine;5’-(4-(fluorosulfonyl)benzoyl)adenosine;5'-(4-Fluorosulfonylbenzoyl)adenosine HCI;5'-O-[4-(Fluorosulfonyl)benzoyl]adenosine;5'-(4-Fluorosulfonylbenzoyl)adenosine HCl;5'-P-FLUOROSULFONYL-BENZOYLADENOSINE HYDROCHLORIDE;5'-(4-FLUOROSULFONYLBENZOYL)ADENOSINE HYDROCHLORIDE
- CBNumber:
- CB7671319
- Molecular Formula:
- C17H17ClFN5O7S
- Molecular Weight:
- 489.86
- MDL Number:
- MFCD00043258
- MOL File:
- 78859-42-4.mol
Last updated:2023-04-23 13:52:06
SAFETY
Risk and Safety Statements
| Hazard Codes | T |
|---|---|
| Risk Statements | 45-20/21/22-36/37/38 |
| Safety Statements | 53-22-26-36/37/39-45-24/25 |
| WGK Germany | 3 |
| F | 10 |
| HS Code | 29349990 |
5'-P-FLUOROSULFONYL-BENZOYLADENOSINE HYDROCHLORIDE price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | F9128 | 5′-(4-Fluorosulfonylbenzoyl)adenosine hydrochloride | 78859-42-4 | 25mg | $547 | 2024-03-01 | Buy |
| Sigma-Aldrich | F9128 | 5′-(4-Fluorosulfonylbenzoyl)adenosine hydrochloride | 78859-42-4 | 100mg | $1680 | 2024-03-01 | Buy |
| Sigma-Aldrich | F9128 | 5′-(4-Fluorosulfonylbenzoyl)adenosine hydrochloride | 78859-42-4 | 500mg | $6630 | 2024-03-01 | Buy |
| TRC | F599218 | 5′-(4-Fluorosulfonylbenzoyl)adenosine hydrochloride | 78859-42-4 | 5mg | $240 | 2021-12-16 | Buy |
| TRC | F599218 | 5′-(4-Fluorosulfonylbenzoyl)adenosine hydrochloride | 78859-42-4 | 0.5mg | $45 | 2021-12-16 | Buy |
5'-P-FLUOROSULFONYL-BENZOYLADENOSINE HYDROCHLORIDE Chemical Properties,Uses,Production
Chemical Properties
Off-white powder
Uses
5′-(4-Fluorosulfonylbenzoyl)adenosine (FSBA) is an ATP analog used to study the mechanism of action of wortmannin. FSBA has been used to study vesicular transport in intestinal cells such as Caco-2, and to covalently modify residues in the nucleotide-binding domains (NBDs) of various ATPases, kinases, and other proteins.
Biochem/physiol Actions
5′-(4-Fluorosulfonylbenzoyl)adenosine helps to recognize adenosine triphosphate (ATP)-binding sites in kinases due to its reaction with nucleophilic amino acids happening within these motifs.
5'-P-FLUOROSULFONYL-BENZOYLADENOSINE HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
5'-P-FLUOROSULFONYL-BENZOYLADENOSINE HYDROCHLORIDE Suppliers
Global( 20)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| NewCan Biotech Limited | +86-0571-86912261 +8613735419629 | sales@newcanbio.com | China | 10027 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 94838 | 76 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51471 | 80 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 13028896684 | sales@rrkchem.com | China | 55717 | 58 |
| BOC Sciences | 16314854226 | inquiry@bocsci.com | United States | 9713 | 58 |
| Energy Chemical | 021-58432009 400-005-6266 | marketing@energy-chemical.com | China | 44941 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24131 | 58 |
| Supplier | Advantage |
|---|---|
| BOC Sciences | 58 |
| TargetMol Chemicals Inc. | 58 |
| NewCan Biotech Limited | 58 |
| Aladdin Scientific | 58 |
| J & K SCIENTIFIC LTD. | 76 |
| Sigma-Aldrich | 80 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | 58 |
| BOC Sciences | 58 |
| Energy Chemical | 58 |
| TargetMol Chemicals Inc. | 58 |
78859-42-4(5'-P-FLUOROSULFONYL-BENZOYLADENOSINE HYDROCHLORIDE)Related Search:
5’-(4-(fluorosulfonyl)benzoyl)adenosine
5'-P-FLUOROSULFONYL-BENZOYLADENOSINE HYDROCHLORIDE
ADENOSINE-5'-(4-FLUOROSULFONYLBENZOATE) HYDROCHLORIDE
5'-(4-FLUOROSULFONYLBENZOYL)ADENOSINE HYDROCHLORIDE
FSBA
Adenosine-5μ-(4-fluorosulfonylbenzoate) hydrochloride, FSBA
5'-Fluorosulfonylbenzoyladenosine
5'-FSBA
5'-O-[4-(Fluorosulfonyl)benzoyl]adenosine
5'-p-Fluorosulfonylbenzoyl adenosine
5'-(4-Fluorosulfonylbenzoyl)adenosine HCI
5'-(4-Fluorosulfonylbenzoyl)adenosine HCl
78859-42-4
C17H16FN5O7SHCl
C17H16FN5O7SHCI
Nucleoside Analogs
Nucleosides, Nucleotides, Oligonucleotides
Organic Covalent Modifiers
Enzymes, Inhibitors, and Substrates
Enzyme Inhibitors
Enzyme Inhibitors by Type
BioChemical
Biochemicals and Reagents